Article / Review Article
1Talwar Research Foundation, New Delhi, India.
2National Institute of Immunology, New Delhi, India.
3Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India.
4Department of Biochemistry, Indian Institute of Science, Bangalore-560012, India.
G P Talwar, Director Research, Talwar research Foundation, E-8, Neb Valley, New Delhi-110068, India.
10 June 2020 ; 15 July 2020
Based on atypical cultivable non-pathogenic mycobacteria, a vaccine with immunoprophylactic cum immuno-therapeutic properties against leprosy was developed by us many years back. The gene sequence of Mw (code word) is now known and it has been named as Mycobacterium indicus pranii (MIP). Besides Leprosy, MIP has also remarkable capabilities for treatment of tuberculosis including category II, “Difficult to treat” tuberculosis. What is further impressive is its ability to cure ugly ano-genital warts. It has therapeutic action against Myelomas. MIP activated T cells and cytokines, particularly ƴ-interferon play a major role in action of MIP against cancer cells. Combination of MIP with cyclophosphamide improves anti-tumour activity.
MIP is a potent invigorator of immune responses and is being employed as an adjuvant in a potential Birth Control Vaccine against hCG, currently under development. MIP is approved by the Drugs Controller General of India & US FDA. It is licensed to a company for availability to public in India and elsewhere in the world. Very recently, a trial has been launched by CSIR, Ministry of Science and Technology on the utility of Mw (MIP) to cure those individuals who are infected with Corona and protect family members if possible by immuno-prophylaxis.
Key words: Mw, MIP, Immunotherapy, Immunoprophylaxis, Adjuvant
On this Easter Sunday, when I (GPT) am starting to write this manuscript, my thoughts go to the people who become victims to this malady, leprosy. It is a disfiguring disease to which luckily few (about 1% in India) fall victims. The causative organism, M. leprae was discovered in Norway about two hundred years back by Armauer Hansen. It can replicate rather slowly (doubling time is about 13 days) in a suitable host cell. It is a mycobacteria surrounded in mystery. In 1970, a team from World Health Organisation (WHO) headed by Dr. Howard Goodman visited me in the Biochemistry Department of the All India Institute of Medical Sciences, New Delhi which I was heading. They wanted me to take up the Headship of a WHO Research & Training Centre in Immunology for India and South East Asia. I was reluctant. They told me that India has the world’s largest number of Lepers in the world. Are we expecting Americans to come and solve this problem? This shocked and shamed me and so I signed the papers without delay.
I knew nearly nothing of leprosy. I spent the next two summer vacations along with two talented students, A.D. Krishnan and Vijaylaxmi Mehra, in a Danish Save The Children Leprosy Home in Orissa to investigate why people fall victims to this disease. This work is reported in several publications. A review of this work is also published [1]. The nature of the immune deficit of those falling victims to this disease is their inability to react to some key antigens of M. leprae. Their immune system is otherwise competent to react and prevent various other prevailing infections, such as cholera, typhoid, etc. An experiment whose results are summarized in Table 1, shows the inability of T cells from multibacillary lepromatous leprosy patients to react with M. leprae to generate signals blocking the multiplication of M. leprae in monocyte-derived macrophages, (indicated by the incorporation of 3H-thymidine), whereas the T cells from Tuberculoid (paucibacillary) leprosy patients are competent to generate signals limiting the multiplication of M.leprae.
Monocyte-derived macrophages as host cells for M. leprae and 3H-thymidine incorporation as a sensitive measurable experimental way to quantitate DNA synthesis as a prerequisite to multiplication was established with the help of my student A.D Krishnan [2]. Macrophages do not have apparently any defect; they can originate from either LL, TT leprosy patients or normal human beings. It is the T cells that make the difference. Those from multibacilliary LL patients are unable to generate cytokines influencing the multiplication or inhibition of the multiplication of indwelling M. leprae. It may be mentioned that monocyte derived macrophages do not multiply in-vitro and do not incorporate any 3H-thymidine into their DNA. This key experiment reported in Table 1 illustrates the nature of defect in leprosy patients.
| Patient No. | Clinical status | CPM 3H-thymidine incorporated per 5×105 phagocytic cells | |
|---|---|---|---|
| Macrophages+Lymphocytes+M.leprae | Macrophages+M.leprae | ||
| 1 | LL | 36,458 | 45,628 |
| 2 | LL | 53,929 | 59,596 |
| 3 | LL | 52.354 | 83,476 |
| 4 | TT | 6,332 | 54,969 |
| 5 | TT | 32 | 78,447 |
| 6 | TT | 381 | 26,260 |
Table 1: Mycobacterial multiplication in cultivated macrophages derived from peripheral blood monocytes of Leprosy patients [2]
LL: Lepromatous multibacillary Leprosy; TT: Tuberculoid paucibacillary form of Leprosy
A Delayed Hypersensitivity Skin Test, a visible reaction to crushed M.leprae (Lepromin), is invariably performed by clinicians to classify the patient. Tuberculoid leprosy TT patients give a positive Lepromin reaction, whereas LL, the multibacillary lepromatous patients are negative to Lepromin. Immunization of BL, LL patients with MIP interestingly converted a fairly high percentage of BL-LL, patients to Lepromin positivity status (Fig. 1), which otherwise never happens.
What was further encouraging was the lasting nature of this conversion from Lepromin negativity to Lepromin positivity. Dr. Chaudhary at the School of Tropical Medicine Kolkata, conducted investigations in 32 Drugs-cured leprosy patients. While multidrugs treatment had rendered them bacteria free, Drugs were unable to render them Lepromin positive. After immunization of these patients with autoclaved MIP, 20 of them became Lepromin positive [3] lessening their chance of getting the disease on re-exposure to M. leprae.
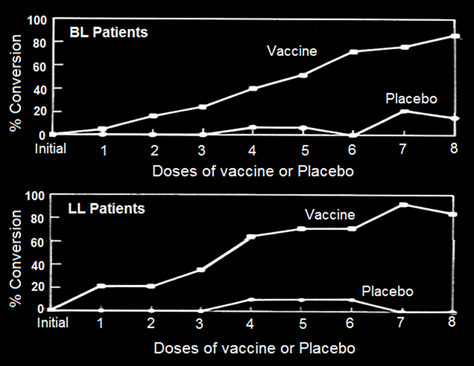
Figure 1: Conversion of lepromin status of Borderline lepromatous (BL)/Lepromatous (LL) patients treated with either MDT alone or MDT+ MIP [4].
Treatment with the multi-drug regime usually takes about 2 years or more for rendering BL, LL patients, bacteria free. Some patients are extremely slow responders. Fig. 2 shows two cases in whom immunization with MIP hastened significantly the recovery [5].
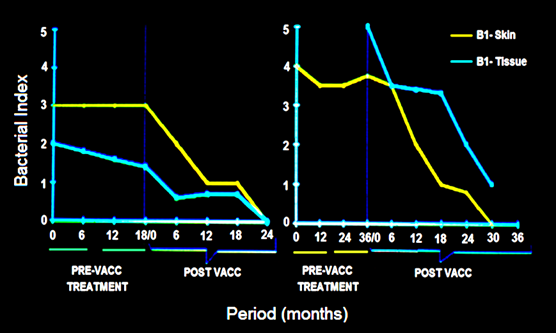
Figure 2: Synergistic effect of Mw vaccine in slow responders to drugs in 2 patients. In both, the Bacterial index started declining on immunization with vaccine [5].
Our work to find appropriate non-pathogenic cultivable mycobacteria was done on 16 atypical mycobacteria obtained from various collections. These were all coded. The one, which eventually came out as a worthy candidate for a vaccine was Mw. Having proved its worth, a decision was taken to determine the gene sequence of Mw. This was done in three laboratories, headed by Prof. Seyed Hasnain, Prof Anil Tyagi and Prof Akhilesh Tyagi: Their findings are reported in [6, 7]. Being given, that it was hitherto an undescribed mycobacteria, it was named as Mycobacterium indicus pranii (MIP); Pran is my family name and NII is the National Institute of Immunology, of which I was the Founder Director and from where a lot of basic work and clinical trials were conducted. Fig. 3 gives an Atomic Force Microscopy perspective of MIP.
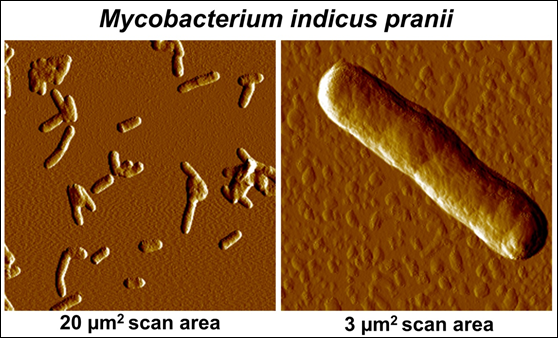
Figure 3: Atomic Force Microscopic imaging of MIP.
Immunization of patients with MIP in addition to the usual approved multidrug treatment hastened bacterial clearance and shortened the period of recovery. What was remarkable was the lack of blemishes and usually ugly lesions normally not cleared by Drugs alone. Fig. 4 shows few patients, who received MIP, besides the Drugs. On recovery many of them looked normal as if they never had leprosy, unlike leprosy patients treated with drugs alone.
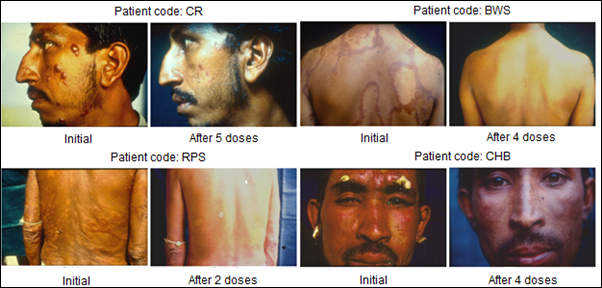
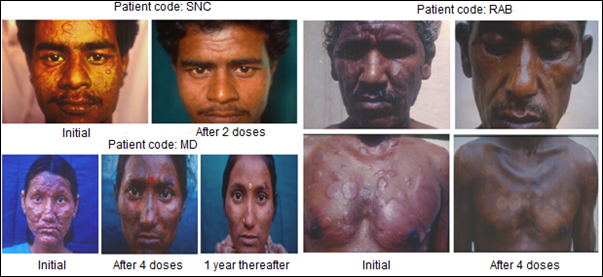
Figure 4: Some representative cases of LL/BL multibacillary patients treated with MDT plus Mw (Mycobacterium indicus pranii) [8].
Currently the entire world is besieged by infection with the Corona-19 virus causing enormous deaths in USA, Italy, Spain, Iran and many countries of the world. Many states in India, specially Maharashtra and Delhi have fairly high infections and deaths. No specific drug or vaccine exists for this infection. The Director General of Council of Scientific Research (CSIR), The Ministry of Science and Technology with the approval of concerned authorities has launched the use of autoclaved Mw (MIP) manufactured by M/s Cadila Pharma company to which it was licensed by me, when I was the Director of the National Institute of Immunology and renewed by the current Director Dr. Amulya Panda. They are selling several preparations of autoclaved MIP (Mw) under different names for various purposes. Their preparation marketed as Sepsivac is fairly effective for immuno-therapy of Gram-negative sepsis. This will be given to patients infected by Corona-19 with the hope that their recovery will be expedited. It will also be given to family members and contacts with the expectation that they do not become victims of this dreadful infection.
We developed a vaccine against human chorionic gonadotropin (hCG), which was astonishingly very effective in preventing pregnancy in sexually active fertile women without derangement of ovulation, hormonal profiles and menstrual regularity [9]. A genetically engineered version of this vaccine has now been prepared and passed on to M/s Bharat Biotech for manufacture under GMP conditions. This vaccine includes MIP as an adjuvant, which enhances substantially antibody response as is evident in Fig.5.
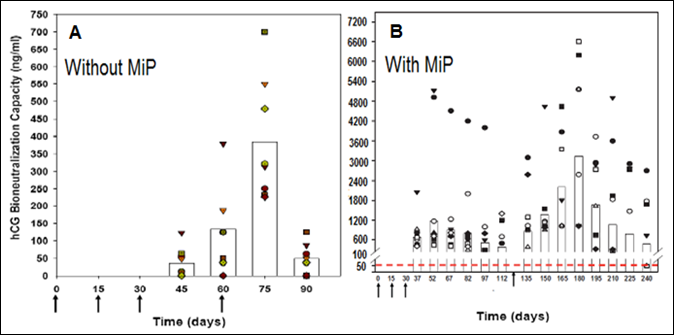
Figure 5: Enhancement of antibody response to hCGβ-LTB vaccine in Balb/c mice by MIP. Mice were immunized intra-muscularly with 2μg of the vaccine adsorbed on alum with or without MIP. Primary immunization consisted of 3 injections given at fortnightly intervals followed by a booster on day 60 or 120. The symbols represent the titres in a given mouse. Bars give the geometrical means [10].
Tuberculosis is a major disease in many parts of the world. A vaccine was developed by Drs Calmette and Guerin at Institut Pasteur Paris about 100 years back based on a Bacillus named after them Bacillus Calmette Guerin (BCG). BCG is employed in India (and elsewhere) for immunizing children. However it has genetic restrictions of response. It is effective somewhere and not effective elsewhere. Thus in India there is need to supplement BCG with additional immunization with another vaccine. Our work shows that combined or subsequent immunization of mice or guinea pigs with MIP enhances substantially protection against Mycobacterium tuberculosis (Fig.6).
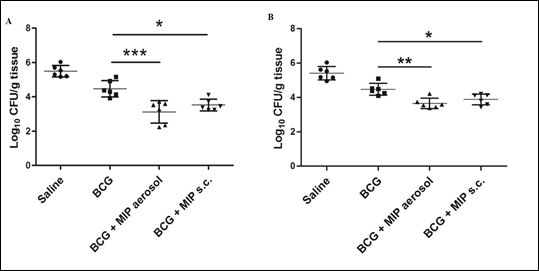
Figure 6: Bacterial loads at 4 and 8 weeks after M.tb challenge. Bacterial load in different groups was evaluated in lungs after 4 weeks (A) and 8 weeks (B) of M.tb challenge. Significantly reduced colony forming units (CFU) per gram of tissue were found in ‘BCG-MIP’ regimen as compared to ‘only BCG’ vaccinated group. Data represents the mean CFU ± SD of six guinea pigs in each group. [*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001].
The current multi-drug regimen in operation includes rifampicin, isoniazid, pyrazinamide and ethambutol. It usually results in poor compliance, as these drugs have to be taken for a minimum of 6-9 months. Although, new cases of TB can be treated by currently available drugs but patients who default on therapy run risk of relapse and drug resistance. Multipronged strategy is needed to effectively eliminate the actively multiplying as well as persistent mycobacteria from the infected lungs.
During the course of M.tb infection, the Th1 response is suppressed and Th2 response gets enhanced which helps in the survival of M.tb and results in progression of disease. An immunotherapy strategy which could boost the Th1 immune response in M.tb infected patients can be effective in clearance of bacteria as it would act synergistically with chemotherapy. Efficacy of MIP as an adjunct to standard chemotherapy for TB was evaluated in animal models, given by aerosol or subcutaneous route. MIP-immunotherapy by aerosol route induced increased infiltration of antigen specific lymphocytes and macrophages in the M.tb infected lungs, which resulted in enhanced reduction of bacterial load in a synergistic way along with chemotherapy. Moreover, there was less pulmonary pathology as compared to ‘drugs only’ treated group [11]. In the late stage of ‘drugs+MIP immunotherapy’ treatment, a balance between inflammatory and suppressive immune response was observed, which controlled initial inflammatory reaction and helped in restoration of normal lung tissue. Recent study by our group suggests that autophagy induced by MIP plays an important role in reversal of phagosome maturation block in M.tb infected macrophages [12]. Combined results of different studies suggest that MIP can be potentially very useful in eliminating the persistent bacteria when given as adjunct to chemotherapy. MIP immunotherapy by intranasal route can play a crucial role in early induction of protective immune response in the lungs.
In many countries including India, BCG is given along with several other vaccines for immunization of children. BCG protects against childhood tuberculosis but is poorly effective in adults. Significant protection against paediatric TB has been observed after BCG vaccination in endemic areas. Prime-boost vaccination is the most practical strategy for control of TB. MIP shares a large number of antigens with M.tb as well as BCG and also has shown higher protection as compared to BCG in animal models of TB. With this background, efficacy of MIP was evaluated as a booster to BCG vaccine in guinea pigs. Booster of MIP enhanced the BCG induced immune response and resulted in higher protection as compared to ‘only BCG’ immunized group (Fig. 6). Reduced pulmonary pathology e.g. less number of lesions, cavities and hemorrhagic spots were observed in ‘BCG-MIP’ group as compared to unvaccinated or ‘only BCG’ groups (Fig. 7). MIP booster via aerosol route was more effective in providing protection as compared to subcutaneous route of booster immunization [13]. Th1 and Th17 cytokines were induced at higher level in infected lungs of ‘BCG-MIP’ group as compared to ‘only BCG’ group. Higher frequency of multifunctional T cells with high MFI for IFN-γ and TNF-α were observed in the ‘BCG-MIP’ group after M.tb infection. Findings of the study demonstrated that MIP can be given as a booster to the BCG vaccine during childhood for efficient protection against TB.
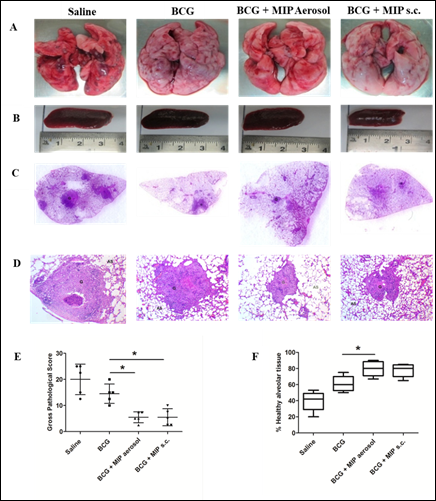
Figure 7: Pulmonary pathology at 4 weeks after infection.Shown are the representative images of lungs (A) and spleen (B) of guinea pigs from different groups at 4 weeks post infection. The presence of pathological lesions like nodules, hemorrhagic spots and cavities in the lungs were macroscopically examined. Spleen size was also measured and compared among the groups. (C) Pictures showing H&E stained whole lung sections. (D) H&E stained sections visualized at 40 X magnification. (E) Gross pathological score and (F) Percent healthy alveolar tissue in infected lungs. Data represents the mean ± SD. [*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; comparisons were made with ‘only BCG’ immunized group].
In 1990s, protective efficacy of MIP against leprosy was evaluated in a population of about 30,000 people in more than 250 villages in Kanpur Dehat in a randomized placebo controlled study. Healthy household contacts of leprosy patients who had no sign of TB disease were given MIP vaccine/placebo. After follow-up of 13 years, retrospective analysis of the data shows that the incidence of tuberculosis was reduced significantly in the MIP vaccinated group as compared to placebo group [14]. Further higher protection of MIP was observed in the subgroup vaccinated with BCG after birth suggesting that MIP booster dose could enhance the protective efficacy of BCG.
In one of the recently concluded clinical trial, MIP was given as an adjunct to chemotherapy in Cat II pulmonary TB patients (Table 2).
| Treatment Description | Cured | Cured (%) |
|---|---|---|
| MIP + MDT (n =49) | 48/49a | 97.96 |
| MDT alone (n=27) | 21/27b | 77.77 |
Table 2: Outcome of the additive effect of MIP in comparison to MDT alone for therapy of Cat II “Difficult to treat” tuberculosis patients.
Notes: aOne patient defaulter for 6 doses, sputum negative after intensive phase; b Six patients- No effect of therapy
Abbreviations: MIP : Mycobacterium indicus pranii; Mw: Mycobacterium w; MDT: multidrug therapy
This was a double blind, placebo controlled, multi-centric trial where MIP demonstrated significantly higher cure rate in “difficult to treat” cases i.e. those with high bacillary load, drug-resistance and/or with bilateral cavities. At 4 week after start of immunotherapy along with standard drugs, sputum culture conversion was observed in significantly higher number of patients (67.1%) given adjunct therapy of MIP as compared to the placebo (57%) group (p = 0.0002). Early sputum conversion in the MIP group suggested that it played an important role in the clearance of M.tb. This has important implications in controlling the spread of M.tb as live bacteria in the sputum are major contributors for high incidence of TB. Recently a Phase III, double-blind, placebo controlled clinical study has started to evaluate efficacy of MIP in preventing TB in healthy household contacts of pulmonary TB patients.
Mycobacterium indicus pranii (MIP) vaccine is promising for treatment of warts though the exact mechanism by which MIP acts is not known. It is postulated that MIP mounts a strong Th1 mediated immune response, and helps clear HPV, which otherwise evades immune response [15-17].
Patients of anogenital warts, were given MIP initially intradermaly in deltoid area of both sides followed two weeks later by weekly intralesional injections into warts. Complete clearance was seen in 8 out of 9 patients with time for complete clearance ranging from 2 to 12 weeks [16]. Fig 8 and 9 give representative photographs showing clearance of ano-genital warts by MIP.
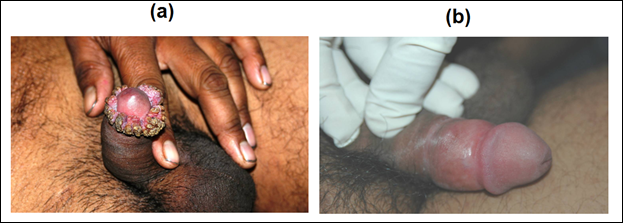
Figure 8: Effect of MIP on ugly anogenital warts. (a) A patient with giant condylomata. (b) The lesions completely subsided with intralesional immunotherapy with MIP.
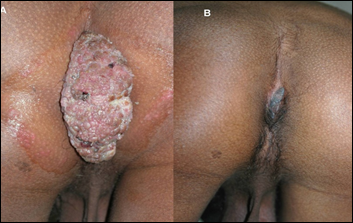
Figure 9: Action of MIP on ugly ano-genital warts (A) Before treatment (B) After treatment with MIP.
Complete clearance in 83% patients of extensive cutaneous warts was seen with MIP vaccine with a mean time of complete clearance of 9.7 weeks (Fig.10) [17]. A study on 30 patients with cutaneous warts at difficult to treat sites were given intralesional Mw vaccine with complete resolution seen in 93.33% patients [18].
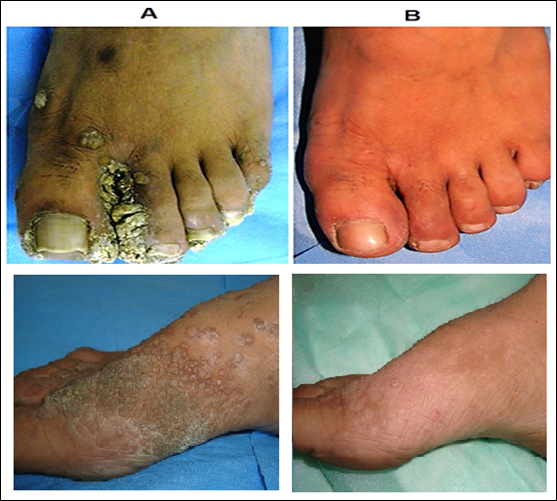
Figure 10: Cure by MIP of warts on feet. (A) Before treatment and (B) After 5 months of treatment with MIP.
A study comparing intradermal Mw vaccine with intradermal purified protein derivative (PPD) of tuberculin antigen in patients of multiple warts showed that MIP vaccine was much more effective than PPD [19]. In 66 patients of refractory extragenital warts, cryotherapy was compared with intralesional MIP vaccine. MIP vaccine had an advantage over cryotherapy in the clearance of distant warts [20].
Immunotherapy with MIP vaccine has proved to be an effective treatment modality for cutaneous warts and has potential as a first line treatment.
Mycobacteria have been known to induce anti-tumor responses ever since the pioneering studies by William Coley and the roles of different mycobacteria are documented [21]. The efficacy of MIP as an anti-tumor agent has been observed in multiple systems. MIP reduces tumours caused by B16F10 melanoma cells. MIP treated mice display higher Th1 responses and lower number of regulatory T cells [22]. We observed that a single intra-dermal injection of MIP, in a dose dependent manner, reduces the subcutaneous growth of SP2/0 myeloma in vivo [23]. The i.d. route of injection is effective. Two cytokines, IL12 and IFNγ, play an important role for anti-tumor immunity [24]. Also, MIP treatment reduces IL6, a known pro-survival factor for tumors [25].
The role of T cells in anti-tumor responses is well recognized [26]. Stimulation of splenocytes from MIP-immunized mice with tumor lysates led to reduction of proliferation of tumor cells. Tumor antigens stimulated the proliferation of specific anti-tumor T cells which kill the tumor cells. Notably, this killing was dependent on CD8+ T cells and was tumor-specific. Also, stimulation of MIP-immunized splenocytes greatly increased the production of IL2 and IFNγ in response to tumor lysates. CD4+ T cells were found to be primarily responsible for the production of cytokines. The data demonstrated that MIP induces a Th1 priming environment which triggers the generation of anti-tumor T cells that boost immunity and reduce tumor cell growth (Fig. 11).
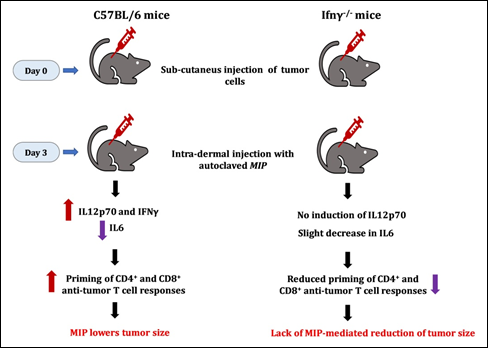
Figure 11: MIP-mediated anti-tumour responses are dependent on Gamma Interferon. No decline of tumor growth was seen in γ interferon negative mice.
It was of interest to investigate the action of MIP on various tumours. For this purpose, the highly invasive EL4 thymoma tumour was employed. The role of IFNγ was investigated using C57BL/6 and Ifng-/- mice. Treatment with MIP reduced the growth of EL4 tumour cells; however, no decline was observed in mice lacking IFNγ (Fig. 11). These observations reveal the importance of IFNγ in mediating the anti-tumor action of MIP. The direct role of MIP-induced anti-tumor lymphocytes was addressed in NOD-SCID mice, which lack lymphocytes. Both Sp2/0 and EL4 tumors grew well in this mouse strain, the efficacy of MIP in reducing tumor growth was not observed [23].
Anti-tumor therapy using combination of MIP and cyclophosphamide
An observation from our studies was that MIP reduces tumor growth, but it was not complete. Also, the efficacy of MIP is lost at later stages of tumor growth. Most likely, the tumor cells overtake the host immune response induced by MIP. These observations led us to investigate the role of other anti-tumor compounds together with MIP. One such compound is cyclophoshamide which reduces the activity of regulatory T cells and enhances T cell responses [27]. MIP administered alone post-inoculation of Sp2/0 cells did not significantly reduce tumor volume nor did cyclophosphamide alone at low doses i.e. 1-15 mg/Kg body weight. However the combination therapy with low doses of cyclophosphamide, administered one day after MIP treatment was observed (Fig. 12).
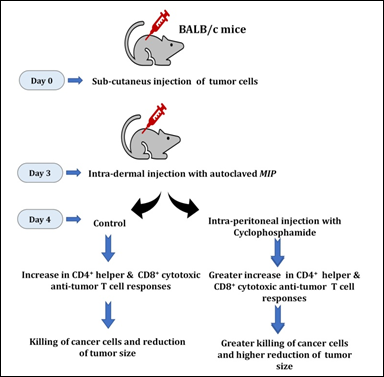
Figure 12: The combination of MIP and cyclophosphamide is potent in lowering the growth of tumors in vivo. Intra-dermal injection of MIP stimulates anti-tumor specific CD4+ and CD8+ T cells to lower tumor growth. The combination of MIP and cyclophosphamide was highly efficient in lowering the growth of tumors.
This combination therapy leads to high amounts of IL12p70 and IFNγ. On the other hand, low levels of IL6, TGFβ, IL1β and IL10 are seen. The synergistic treatment was effective for both Sp2/0 and EL4 tumor model systems, including administration at later stages of tumor development (http://www.google.com/patents/US8367075; United States Patent No. 8,367,075 B2).
Overall, our laboratory at the Indian Institute of Science, Bangalore, has carried out atomic force imaging and studied mechanisms involved in the immunotherapeutic effects of MIP. We have shown the role of MIP in the induction of Th1 cytokines and the stimulation of anti-tumor CD4+ and CD8+ T cell responses. In addition, the efficacy of MIP together with cyclophosphamide in significantly reducing tumor growth in mice was observed. Further studies are required to identify the MIP encoded molecules that are responsible for the adjuvant efficacy of MIP in lowering tumors [28]. Studies are also required to evaluate and understand the mechanisms, e.g. roles of signalling and effector molecules [29, 30] by which MIP modulates immune responses and is beneficial to the host. Overall, these studies are likely to enhance our understanding of the host immune network and may improve the quality of life.
Funding: The work reviewed in this article received research grants from the Indian Council of Medical Research and the Department of Biotechnology Government of India.
Authors have no conflicts of interest
- Talwar GP (1999) “An immunotherapeutic vaccine for multibacillary leprosy”. Int Rev Immunol 18(3): 229-249.
- Talwar GP, Krishnan AD, Jha P, Mehra V (1974) “Intracellular Growth of an obligatory parasite Mycobacterium leprae. Host Bacterial Interactions”. Biochimie 56: 231-237.
- Chaudhuri S, Fotedar A, Talwar GP (1983) “Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium W”. Int J Lepr & Other Mycobact Dis 51(2):159-68.
- Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, Parida SK, Reddi PP, Ahmed N, Saini V, Gupta S (2017) “Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties”. Biologics: Targets & Therapy 11:55-63.
- Zaheer S. A, Mukherjee A, Ramesh V et al (1995) “Immunotherapy benefits multibacillary patients with persistently high bacteriological index despite long term multidrug therapy”. Immunol Infect Dis 5:115-122.
- Saini V, Raghuvanshi S, Talwar GP, Ahmed N, Khurana JP, Hasnain SE, Tyagi Akhilesh K, Tyagi Anil K (2009) “Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species”. PLoS One 4: e6263.
- Talwar GP, Ahmed N, Saini V (2008) “The use of the name Mycobacterium W for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): a suggestion”. Infect Genet Evol 8: 100-101.
- Talwar G.P. (2014) “Leprosy is in principle eradicable – a possible approach”. Curr Sci 106: 1344-45.
- Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, Dhall K, et al (1994) “A vaccine that prevents pregnancy in women”. Proc Natl Acad Sci USA 91:8532-36.
- Purswani S, Talwar GP, Vohra R, Pal R, Panda AK, Lohiya NK, Gupta JC (2011) “Mycobacterium indicus pranii is a potent immunomodulator for a recombinant vaccine against human chorionic gonadotropin”. J Reprod Immunol 91: 24-30.
- Gupta A, Ahmad FJ, Ahmad F, Gupta UD, Natarajan M, Katoch V and Bhaskar S (2012) “Efficacy of Mycobacterium indicus pranii Immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung”. PLoS One 7(7):e39215.
- Singh B, Saqib M, Gupta A, Kumar P and Bhaskar S (2017) “Autophagy induction by Mycobacterium indicuspranii promotes Mycobacterium tuberculosis clearance from RAW 264.7 macrophages”. PLoS One 12(12): e0189606.
- Saqib M, Khatri R, Singh B, Gupta A and Bhaskar S (2016) “Mycobacterium indicus pranii as a booster vaccine enhances BCG induced immunity and confers higher protection in animal models of tuberculosis”. Tuberculosis 101: 164e173.
- Katoch K, Singh P, Adhikari T, Benara SK, Singh HB, Chauhan DS, Sharma VD, Lavania M, Sachan AS and Katoch VM (2008) “Potential of Mw as a prophylactic vaccine against pulmonary tuberculosis”. Vaccine 26: 1228-1234.
- Tindle, R (2002) “Immune evasion in human papillomavirus-associated cervical cancer”. Nat Rev Cancer 2: 59–64.
- Gupta S, Malhotra AK, Verma KK, Sharma VK (2008) “Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study”. J Eur Acad Dermatol Venereol 22:1089-93.
- Singh S, Chouhan K, Gupta S (2014) “Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts”. Indian J Dermatol Venereol Leprol 80:509-14.
- Meena JK, Malhotra AK, Mathur DK, Mathur DC (2013) “Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: uncontrolled open study”. JAMA Dermatol 149:237-9.
- Kumar P, Dar L, Saldiwal S, Varma S, Datt Upadhyay A, Talwar D, Sharma VK, Verma KK, Dwivedi SN, Raj R, Gupta S (2014) “Intralesional injection of Mycobacterium w vaccine vs imiquimod, 5%, cream in patients with anogenital warts: a randomized clinical trial”. JAMA Dermatol 150:1072-8.
- Chandra S, Sil A, Datta A, Pal S, Das NK (2019) “A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts”. Indian J Dermatol Venereol Leprol 85:355-366.
- Podder S, Rakshit S, Ponnusamy M, Nandi D (2016) “Efficacy of bacteria in cancer immunotherapy: special emphasis on potential of mycobacterial species”. Clinical Cancer Drugs 3: 100-108.
- Ahmad F, Mani J, Kumar P, Haridas S, Upadhyay P, Bhaskar S (2011) “Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice”. PLoS One 6: e25424.
- Rakshit S, Ponnusamy M, Papanna S, Saha B, Ahmed A, Nandi D (2012) “Immunotherapeutic efficacy of Mycobacterium indicus pranii in eliciting anti-tumor T cell responses: critical roles of IFNγ”. Int J Cancer 130: 865-875.
- Dranoff G (2004) “Cytokines in cancer pathogenesis and cancer therapy”. Nat Rev Cancer 4: 11-22.
- Ara T, Declerck YA (2010) “Interleukin-6 in bone metastasis and cancer progression”. Eur J Cancer 46: 1223-1231.
- Nandi D, Pathak S, Verma T, Singh M, Chattopadhaya A, Thakur S, Raghavan A, Gokhroo A, Mahantesh V (2020) “T cell costimulation, check point inhibitors and anti-tumor responses”. Journal of Biosciences 45: 50.
- Taieb J, Chaput N, Schartz N, Roux S, Novault S, Ménard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jèze G, Lemonnier F, Zitvogel L (2006) “Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines”. J. Immunol 176: 2722-2729.
- Saqib M, Khatri R, Singh B, Gupta A, Bhaskar S (2019) “Cell wall fraction of Mycobacterium indicus pranii shows potential Th1 adjuvant activity”. Int Immunopharmacol 70:408-416.
- Halder K, Banerjee S, Ghosh S, Bose A, Das S, Chowdhury BP, Majumdar S (2017) “Mycobacterium indicus pranii (Mw) inhibits invasion by reducing matrix metalloproteinase (MMP-9) via AKT/ERK-1/2 and PKCα signaling: A potential candidate in melanoma cancer therapy”. Cancer Biol Ther 18: 850-862.
- Kumar P, Das G, Bhaskar S (2019) “Mycobacterium indicus pranii therapy induces tumor regression in MyD88- and TLR2-dependent manner”. BMC Res Notes 12:648.













