Article / Conference Presentation
Research and Development Manager, CIPREC, Argentina.
Dr. Fernando Guerlloy,
Research and Development Manager, CIPREC
Argentina.
25 August 2025 ; 8 September 2025
Clinical research not only advances therapeutic innovation but also strengthens healthcare systems and improves patient experience. This study integrates two perspectives: the perceptions of patients participating in clinical trials and the effect of digitalization on research protocol management. A survey was conducted among 2,140 patients, comparing routine care with clinical trial participation across five dimensions. In parallel, management metrics were analyzed before and after the adoption of a digital platform. Results demonstrate significantly higher patient satisfaction in clinical trials and substantial improvements in efficiency after digitalization. CIPREC thus represents a replicable model of a modern research center, integrating scientific innovation, public health, and patient-centered care.
Clinical research has traditionally been evaluated in terms of its contribution to therapeutic innovation. However, its role extends beyond drug development: it can reinforce healthcare delivery systems and enhance patients’ perception of quality. In recent years, attention has also turned to the digital transformation of clinical research operations, aiming to improve data quality, efficiency, and scalability.
At CIPREC (Centro de Investigación en Prevención Cardiovascular), located in Buenos Aires, Argentina, we sought to evaluate two aspects in parallel:
- Patient satisfaction and perceptions when participating in clinical trials compared with routine care.
- The impact of digitalization on research protocol management and operational efficiency.
- To assess patient satisfaction and perception regarding their participation in clinical trials.
- To analyze the effect of digitalization on the efficiency and quality of protocol management in a clinical research center.
Patient Survey
A structured satisfaction survey was administered to 2,140 patients between 2023 and 2024 (age range 32–83 years). Patients were asked to compare their experiences in routine clinical care versus participation in clinical research protocols. Five dimensions were assessed: satisfaction, expectations, motivation, organization, and perceived benefit. Responses were scored on a 1–5 scale.
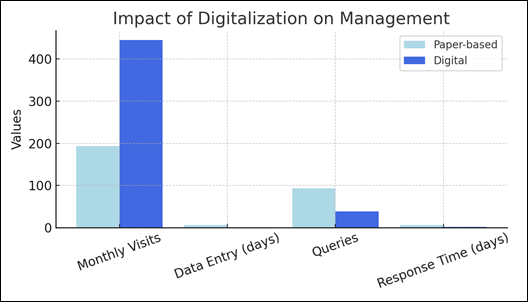
Operational metrics were compared between two distinct periods:
- Period A (2020–2022): paper-based system.
- Period B (2022–2024): Brainmart MED CT digital platform.
Metrics included monthly patient visits, data entry time, number of queries, and query response times.
Patient Satisfaction
Patients reported consistently higher scores in all dimensions when participating in clinical trials:
- Satisfaction: 3.8 vs 2.8
- Expectations: 3.2 vs 2.6
- Motivation: 3.2 vs 2.4
- Organization: 3.9 vs 1.9
(All comparisons p < 0.001).
Transition to digital management produced significant efficiency gains:
- Monthly visits increased from 193 to 445.
- Data entry time decreased from 7 to 1 day.
- Queries dropped from 93 to 39.
- Response times shortened from 7 to 2 days.
These findings highlight two complementary dimensions of clinical research. On the one hand, patients perceive clinical trial participation as more organized, motivating, and satisfactory than routine care. This reinforces the concept that clinical trials not only provide access to innovation but also contribute to improving patient experience.
On the other hand, digitalization has transformed protocol management, enabling a higher patient volume, faster data handling, and fewer errors. These operational improvements are critical for meeting regulatory standards and ensuring data quality.
An important implication is that clinical research centers should be integrated within healthcare systems. When research activities align with clinical practice, both patients and physicians benefit: patients gain access to cutting-edge therapies, and physicians contribute to scientific progress while enhancing quality of care.
- Clinical trials improve patients’ perception of care, adding value beyond therapeutic innovation.
- Digital management significantly increases efficiency and ensures higher data quality.
- CIPREC stands as a replicable model of a modern research center, combining scientific innovation, public health, and patient-centered outcomes.
- Integration of clinical research centers into healthcare systems, together with physician participation, is essential to maximize the impact of clinical research on population health.
Figure 1: Patient perceptions comparing routine care and clinical trials.
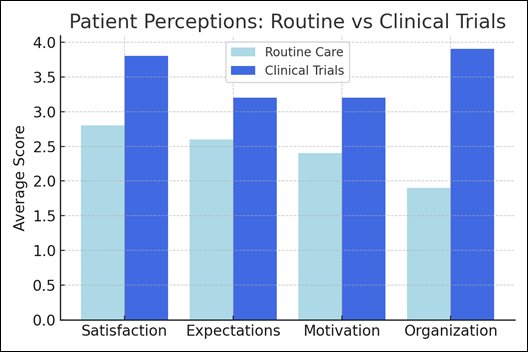
Figure 2: Impact of digitalization on protocol management metrics.