Article / Clinical Image Article
1Division of Nephrology, Cliniques Universitaires Saint-Luc, Brussels, Belgium.
2Laboratory Department, Hematology Laboratory, Cliniques Universitaires Saint-Luc, Brussels, Belgium.
Prof. Nada Kanaan
Division of Nephrology,
Cliniques universitaires Saint-Luc,
Brussels,
Belgium
30 November 2023; 19 January 2024
A woman in her 70s presented four months after receiving a kidney transplant with fever, epigastric discomfort and loss of appetite. She had experienced antibody-mediated rejection ten days after transplantation, successfully treated with high doses of intravenous corticosteroids, plasma exchanges and rituximab. Two months earlier, neutropenia had led to the discontinuation of valganciclovir prophylaxis, administered in the context of a CMV-seropositive donor/seronegative recipient status. Her immunosuppressive therapy included tacrolimus, mycophenolate mofetil and corticosteroids.
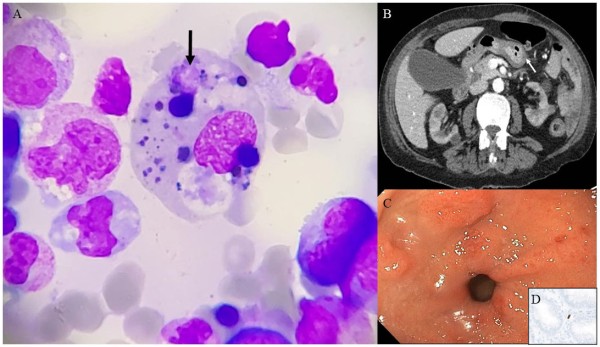
Upon admission, physical examination was notable for pale teguments and epigastric tenderness. Biochemical tests revealed increased C-reactive protein (40 (N < 5) mg/L) with normal fibrinogen, severe cytopenia affecting the three lineages (hemoglobin 7.6 g/dL, neutrophils 670/mm3, platelets 86000/mm3 respectively), hyperferritinemia (8437 (N: 13-150) µg/L), hypertriglyceridemia (4.5mmol/L), transaminitis (ALT (N<35) 36 U/L), and increased LDH. Cytomegalovirus viral-load was extremely high (20×106 IU/ml). Bone marrow aspiration showed hemophagocytosis (arrow) in macrophages (Panel A). CT-scan showed upper limit spleen size and gastric wall swelling (Panel B) with esophagogastroduodenoscopy revealing erosive antral gastritis (Panel C), staining positive for cytomegalovirus on immunohistochemistry (Panel D). Donor-transmitted cytomegalovirus induced hemophagocytic lymphohistiocytosis (HLH) was diagnosed (calculated H score 253; 99% probability).
Secondary HLH is a potentially life-threatening hyperinflammatory hyperferritinemic immune response associated with a cytokine storm caused by aberrantly activated macrophages and cytotoxic T lymphocytes. It is triggered by infections (mainly viruses, but also bacteria, parasites and fungi), malignancies (mainly lymphomas) or auto-inflammatory/autoimmune disorders- in which case it is called macrophage activation syndrome[1].
The diagnosis of secondary HLH is based on clinical presentation in the presence of elevated ferritin and must fulfill the HLH-2004 diagnostic criteria. The HLH-probability calculator (HScore) may help with diagnosis (an HScore ≥250 confers a 99% probability of HLH)[2]. Prompt treatment of the underlying trigger is mandatory for favorable evolution. In our patient, intravenous ganciclovir was initiated and mycophenolate mofetil discontinued. Several weeks of IV treatment were necessary before progressive viral clearance, normalization of biomarkers and clinical improvement.
- Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A et al. Adult haemophagocytic syndrome. Lancet 2014;383:1503-16.
DOI : https://doi.org/10.1016/s0140-6736(13)61048-x - Paul La Rosée, AnnaCarin Horne, Melissa Hines et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. DOI: https://doi.org/10.1182/blood.2018894618













