Article / Case Report
1Department of Vascular Surgery, “Annunziata” Hospital, Cosenza.
2Department of Vascular Surgery, A.O.U. “R. Dulbecco”, 88100 Catanzaro.
3Immunohaematology Section, Annunziata Hospital, 1 Via Felice Migliori, 87100 Cosenza, Italy.
4Interuniversity Center of Phlebolymphology (CIFL), “Magna Graecia” University, 88100 Catanzaro, Italy.
5Department of Medical and Surgical Sciences, Magna Graecia University of Catanzaro, 88100, Catanzaro, Italy.
Raffaele Serra,
Department of Medical and Surgical Sciences, Magna Graecia University of Catanzaro, 88100,
Catanzaro, Italy.
Paolo Perri,
Department of Vascular Surgery,
“Annunziata” Hospital,
Cosenza.
18 October 2024 ; 9 November 2024
Central Venous Catheterization (CVC) through the internal jugular vein is a routine procedure conducted in critically ill patients for hemodynamic monitoring, medication administration, and fluid management both in preparation for and during major surgery and for possible dialysis therapy. We present a case of an 80-year-old patient who went to hospital due to a sudden increase in creatinine levels. Subjected to an attempted CVC placement procedure, she suffered damage to the right common carotid artery with perforation and hemorrhage of the carotid artery. Taken immediately to the operating room, it was decided to repair the common carotid artery endovascularly using a covered stent with an immediate stop to the bleeding.
Carotid Injury, Iatrogenic Injury, Carotid Stenting, hematoma, trauma surgery, blood transfusions, emergency transfusions.
Central Venous Catheterization (CVC) through the internal jugular vein is a routine procedure conducted in critically ill patients for hemodynamic monitoring, medication administration, and fluid management. Despite its generally considered safe, incidents of carotid artery injury frequently occur during the intended catheterization of the internal jugular vein (Agid et al., 2012). If left unaddressed, or if positioned incorrectly, damage to the carotid artery can lead to severe bleeding, neurological impairments, or fatal outcomes. Endovascular interventions, specifically covered stent deployment, have emerged as a superior alternative to traditional surgical repairs for carotid artery perforation, rupture, and other neurovascular emergencies (DuBose et al., 2008). Apart from perforation with hemorrhage or rupture, other morphological injuries such as obstruction, pseudoaneurysm, fistula, and dissection of the carotid artery may necessitate surgical intervention. We present a case involving an 80-year-old female with acute kidney failure who experienced an iatrogenic carotid injury following jugular vein cannulation, and this was successfully managed using an endovascular-covered stent.
An 80-year-old female with a medical history encompassing hypertension and diabetes mellitus presented at the emergency department following an abrupt elevation in blood creatinine levels. Given the need for dialysis treatment, central venous access was imperative. The right internal jugular vein was selected for cannulation due to its accessibility and proximity to the central circulation. The procedure, guided by ultrasound, entailed the patient assuming a supine position with the head slightly turned to the left. After meticulous skin preparation and local anesthesia administration, a 7-French triple-lumen catheter was inserted using the Seldinger technique. Despite encountering challenges, the catheter was successfully placed.
Post-procedure, the patient experienced neck swelling, tenderness on the right side, and dyspnea. Subsequent imaging revealed a significant neck hematoma, leading to the performance of a chest X-ray to eliminate pneumothorax.
Arterial blood gases indicated diminished oxygen saturation, prompting an urgent contrast-enhanced Computed Tomography (CT) angiogram of the neck.
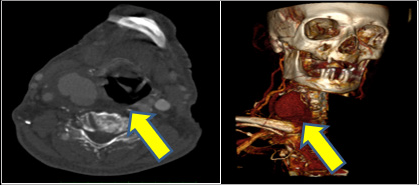 Figure 1: CTA of the neck revealing common carotid injury (transversal view). Yellow arrows shows the hematoma
Figure 1: CTA of the neck revealing common carotid injury (transversal view). Yellow arrows shows the hematoma
This confirmed active blood extravasation and pseudoaneurysm formation from an accidental puncture site in the right common carotid artery. Immediate removal of the central venous catheter and direct manual compression at the bleeding site was executed. Given the intricate nature and location of the injury, an endovascular approach was deemed the most appropriate intervention. The patient was expeditiously transferred to the angiography room for emergency repair. Under local anesthesia, arterial access via the right common femoral artery was established. A diagnostic angiogram validated injury to the middle third of the right common carotid artery with extravasation, leading to the deployment of a covered stent Covera Plus 9x60mm ( BD, Becton, Dickinson and Company, 1 Becton Drive Franklin Lakes, NJ) due to its flexibility and compatibility with the tortuous anatomy.
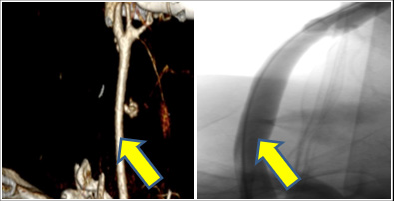
Figure 2: CTA of the neck and Intraoperative angiography shows active bleeding ( yellow arrow)
The stent was meticulously navigated and deployed across the injury site through an 8FR introducer, excluding the pseudoaneurysm and restoring normal carotid artery blood flow without residual leakage. At the end of the procedure, closure of the percutaneous common femoral access using the closure system Angio-Seal 8 (St. Jude Medical Inc. 14901 DeVeau Place Minnetonka, MN).
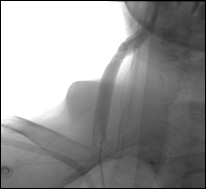 Figure 3: Intraoperative delivery and release phases deployment of Covered Stent in common carotid artery
Figure 3: Intraoperative delivery and release phases deployment of Covered Stent in common carotid artery
The patient underwent blood transfusion with 1 bag of concentrated red blood cells. Post-procedure, the patient was closely monitored in the intensive care unit for 24 hours. To mitigate the risk of in-stent thrombosis, dual antiplatelet therapy (Aspirin 100 mg and Clopidogrel 75 mg) was initiated. The neck hematoma exhibited gradual resolution, and the patient remained neurologically intact without developing complications such as stroke or infection. After three days, the patient was discharged with instructions to continue dual antiplatelet therapy for six months. At the 3-month follow-up, a carotid artery duplex ultrasound revealed favorable stent patency without evidence of restenosis or recurrent pseudoaneurysm formation. The patient reported no neurological symptoms and had resumed his usual daily activities.
Iatrogenic carotid artery injury is a rare but serious complication of diagnostic and therapeutic procedures. The preferred approach for addressing iatrogenic carotid injuries involves endovascular management, as opposed to the management of symptomatic carotid artery stenosis, for which carotid endarterectomy is the treatment of choice. Endovascular approaches to carotid injuries have become increasingly utilized, especially with the growing experience in using endovascular stents for cerebrovascular disease (Hobson et al., 2007). Endovascular stenting has been primarily employed for high extracranial internal carotid lesions, particularly in cases where surgical approaches are challenging and carry a high risk of local and cerebrovascular complications (Maras et al., 2006). Furthermore, endovascular approaches may be particularly beneficial for the treatment of select types of internal carotid injuries, as surgical resection or repair of internal carotid pseudoaneurysms is associated with a high mortality rate (30%) and a high incidence of cerebral complications (Pozzati et al., 1989). Comparing the outcomes of surgical treatment and carotid stenting, the associated mortality rate of carotid stenting is much lower at 0.9% compared to up to 22% for surgical treatment. Additionally, the stroke rates associated with carotid stenting of trauma (3.5%) are comparable to those after operative intervention for carotid injuries (0–21%) and elective stenting for cerebrovascular disease (4.7%) (Ledgerwood et al., 1980; Demetriades et al., 1989; Navsaria et al., 2002; Robbs, et al., 1983). In particular, Du Bose et al. have reported a success rate of 71.3% for endovascular stenting of the internal carotid artery, with only 2.4% of new patients experiencing post-procedural neurological dysfunction.
In the event of rupture, perforation, or dissection of the carotid injury, rapid hemodynamic instability may ensue, necessitating an immediate CT angiogram of the neck to assess the presence of extravasation around the common carotid artery and any extensive hemomediastinum (DuBose et al., 2008).
Employing the femoral approach, angiography can then be utilized to precisely identify the location of the extravasation, following which a balloon-inflatable covered stent can be positioned over the damaged segment (DuBose et al., 2008).
In this case, the quick placement of a covered stent successfully prevented further injury to the carotid artery, eliminated the need for open surgery, and maintained the normal blood flow in the carotid artery. Covered stents are specifically designed to promptly stop bleeding by sealing any defects in the artery, making them highly effective in treating pseudoaneurysms and preventing additional bleeding. There are numerous approaches to emergency transfusion resuscitation but they each center on the early utilization of blood products while allowing for permissive hypotension and minimizing crystalloid administration. In accordance with the literature and good practices in the event of haemorrhage, blood transfusions must be started as early as possible (Farrel et al., 2020).
Endovascular treatment with a covered stent is an effective option for managing iatrogenic carotid artery injuries. It offers a minimally invasive solution with excellent outcomes. In this case, timely diagnosis and intervention prevented significant complications, and the patient recovered uneventfully. This case highlights the importance of early imaging, multidisciplinary collaboration, and appropriate selection of endovascular devices in managing vascular injuries.
- Agid, R., Simons, M., Casaubon, L. K., & Sniderman, K. (2012). Salvage of the carotid artery with covered stent after perforation with dialysis sheath. A case report. Interv Neuroradiol, 18(4), 386-390. DOI: https://doi.org/10.1177/159101991201800404
- DuBose, J., Recinos, G., Teixeira, P. G. R., Inaba, K., & Demetriades, D. (2008). Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma, 65(6), 1561-1566. DOI: https://doi.org/10.1097/ta.0b013e31817fd954
- Hobson, R. W. 2nd. (2007). Randomized clinical trials: impact on clinical practice for symptomatic and asymptomatic extracranial carotid occlusive disease. Perspect Vasc Surg Endovasc Ther, 19(3), 215-9. DOI: https://doi.org/10.1177/1531003507305267
- Maras, D., Lioupis, C., Magoufis, G., Tsamopoulos, N., Moulakakis, K., & Andrikopoulos, V. (2006). Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol, 29(6), 958 –968. DOI: https://doi.org/10.1007/s00270-005-0367-7
- Pozzati, E., Giuliani, G., Poppi, M., & Faenza, A. (1989). Blunt traumatic carotid dissection with delayed symptoms. Stroke, 20(3), 412– 416. DOI: https://doi.org/10.1161/01.str.20.3.412
- Ledgerwood, A. M., Mullins, R. J., & Lucas, C. E. (1980). Primary repair vs ligation for carotid artery injuries. Arch Surg, 115(4), 488–493. DOI:https://doi.org/10.1001/archsurg.1980.01380040110019
- Demetriades, D., Skalkides, J., Sofianos, C., Melissas, J., & Franklin, J. (1989). Carotid artery injuries: experience with 124 cases. J Trauma, 29(1), 91–94. https://pubmed.ncbi.nlm.nih.gov/2911110/
- Navsaria, P., Omoshoro-Jones, J., & Nicol, A. (2002). An analysis of 32 surgically managed penetrating carotid artery injuries. Eur J Vasc Endovasc Surg, 24(4), 349 –355. DOI: https://doi.org/10.1053/ejvs.2002.1736
- Robbs, J. V., Human, R. R., Rajaruthnam, P., Duncan, H., Vawda, I., Baker, L. W. (1983). Neurological deficit and injuries involving the neck arteries. Br J Surg, 70(4), 220 –222. DOI: https://doi.org/10.1002/bjs.1800700412
- Farrel, M. S., Kim, W. C., & Stein, D. M. Emergency Transfusions. Emerg Med Clin North Am, 38(4), 795-805. DOI: https://doi.org/10.1016/j.emc.2020.06.005
- Welling, R. E., Kakkasseril, J. S., & Peschiera, J. (1985). Pseudoaneurysm of the cervical internal carotid artery secondary to blunt trauma. J Trauma, 25(11), 1108 –1110. https://pubmed.ncbi.nlm.nih.gov/4057304/.













