Article / Review Article
1Talwar Research Foundation, New Delhi, India
2National Institute of Immunology, New Delhi, India
3Sir Ganga Ram Hospital, New Delhi, India
4Department of Obstetrics and Gynecology, University of Upasala, Upasala, Sweden
G.P. Talwar
Director Research
Talwar Research Foundation
E-8, Neb Valley
New Delhi-110068
India
22 August 2020 ; 26 September 2020
This article reviews a successful approach to control of cancers promoted by two hormones: Testosterone and Human Chorionic Gonadotropin. The vaccine blocking the production of testosterone, LHRH-DT/TT, the decapeptide linked to either tetanus or diptheria toxoid generates successfully antibodies against both LHRH and tetanus or diptheria, offering additional protection against these infections. It was successful in blocking estrus in dogs and was immunogenic in humans blocking the production of testosterone in males and estrogens in females. It has undergone evaluation in humans suffering from carcinoma of prostate. The vaccine against hCG is composed of hCGβ linked to either TT/DT or heat labile enterotoxin of E. coli. Its beneficial effects have been observed for not only control of unwanted pregnancy in women, but also in myelomas and other cancers.
LHRH is a decapeptide common to both males and females. It is conserved across species. To link LHRH to a carrier, glycine at position 6 of the molecule was replaced by D-Lysine which enabled its linkage to a carrier as illustrated in (Fig. 1).
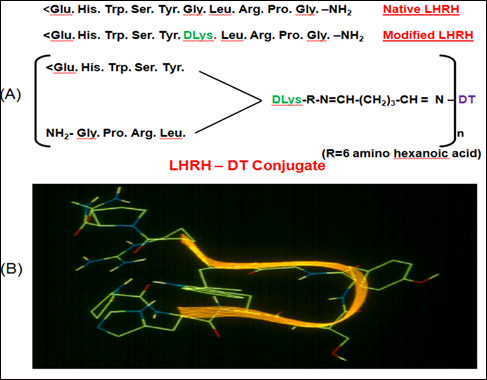
Figure 1: (A) Amino acid sequence of LHRH-DT Vaccine [1]. (B) Structure of LHRH modeled by a knowledge based approach. The molecule takes a bend in the middle to proximate N and C terminal amino acids. Superimposed ribbon drawing highlights the type III b–turn in the hormone [1].
This vaccine was fairly immunogenic and induced effective response without any observable side effects. Given to male rodents, it caused atrophy of Prostate as shown in (Fig. 2).
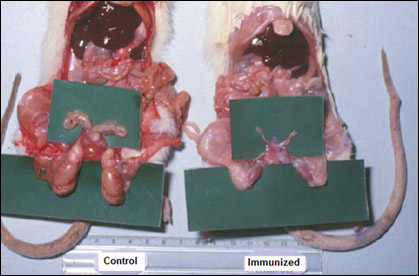
Figure 2: Effect of anti-LHRH vaccine on rat prostate [2]
It could be employed in pet female dogs to control estrus protecting her from getting pregnant (Fig. 3).
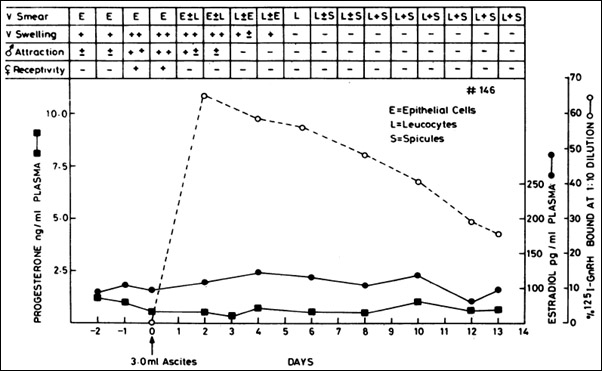
Figure 3: Typical effect of administered anti-GnRH monoclonal antibody given to female dogs in the proestrus stage. The antibody suppressed heat within 48 hr as determined by the four criteria listed in the upper part of the figure. The increase in blood progesterone and estradiol normally occurring during estrus was also prevented. Dashed line shows amount of antibody in circulation on different days after a single i.v. injection of 3 ml of the ascites fluid of clone P81662 [3].
After due Toxicology studies, permission was obtained from Ethics Committees and Drugs Regulatory Authorities for Clinical Trials in patients suffering from Carcinoma of Prostate. The trials were conducted in 28 patients of carcinoma of prostate, 12 each at the All India Institute of Medical Sciences, New Delhi and at Postgraduate Institute of Medical Research and Education, Chandigarh and 4 patients at Urologische Zentrum Salzburg, Austria. Fig. 4 shows the clearance of prostate mass in a patient in Chandigarh.
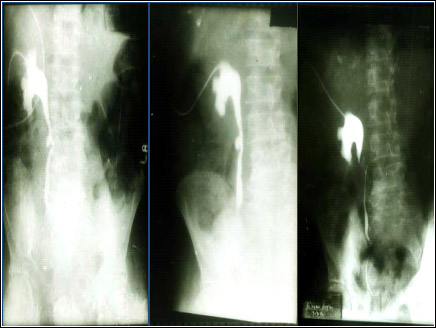
Figure 4: Nephrostograms showing the noticeable reduction of prostatic tissue mass at various stages of immunization with anti-LHRH vaccine [4].
Figure 5 illustrates that induction of anti-LHRH antibodies led to marked reduction of testosterone and PSA (Prostatic Specific Antigen) in a 81 year old patient in Austria. Boosters with the vaccine given in declining stage of antibody titres, boosted the antibody titres accompanied by a decline of PSA.
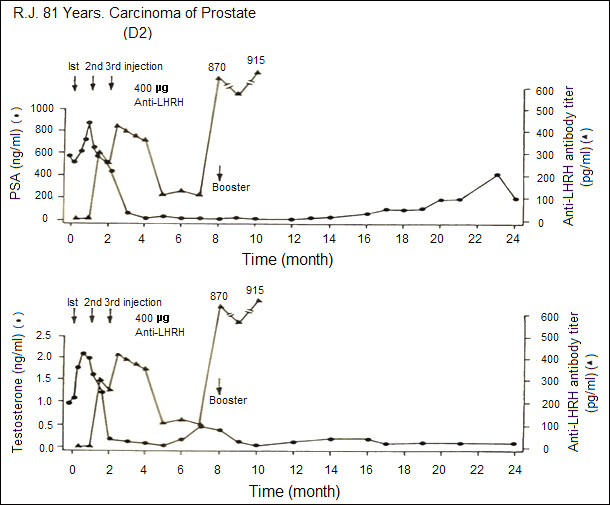
Figure 5: Effect of anti-LHRH vaccine on a patient with advanced carcinoma of prostate. With the generation of antibodies, testosterone and prostatic specific antigen (PSA) levels fall and stay low for several months [4].
Table 1 summarizes the observations on 12 patients immunized with the LHRH vaccine at AIIMS. 6 Patients received a dose of 200 μg of the vaccine per injection and 6 patients received 400 μg dose. These observations point out to the benefit that vaccination with LHRH vaccine can give to such patients.
| Effect of immunization | 200 μg (n=6) | 400 μg (n=6) |
|---|---|---|
| Clinically Stable/ Improvement in Symptoms | 4 | 5 |
| Reduction in Prostatic Size/ Hardness | 1 | 3 |
| Reduction in Acid Phosphatases | 1 | 4 |
Table 1: Observations in clinical trials conducted at AIIMS in patients of carcinoma of prostate after immunization with either 200 μg or 400 μg of anti-LHRH vaccine. Vaccine was administered as 3 primary injections at monthly interval followed by a booster at 8th month [4].
LHRH vaccine is now being converted into a genetically engineered recombinant vaccine so that it could be made at a low cost for availability to Public. Furthermore, its utility for Breast cancer is under examination in our research lab.
We made a vaccine against hCG which was based on HSDTT/ DT (a hetero species dimer, βhCG associated with αoLH). The β subunits of hCG can associate with alpha subunit of ovine LH to generate a dimer, which is more immunogenic than βhCG [5]. The dimer-TT/DT went through Toxicology studies and obtained regulatory approvals for going on human clinical trials. After its safety was established by Phase I trials [5, 6], it was evaluated for its ability to protect women of proven fertility from becoming pregnant. The historic Phase II trials were conducted in 3 major Institutions. All women immunized with the vaccine generated anti-hCG antibodies, which protected women having antibody titres of 50 ng/ml and above from becoming pregnant. (Fig. 6) shows the antibody response in 4 women.
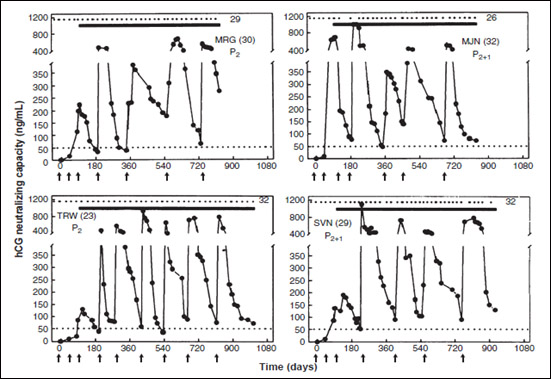 Figure 6: Anti-hCG response to the HSD vaccine in 4 sexually active women of proven fertility. MRG 30 year old and TRW 23 year old had 2 children each; HJN 32 year & SVN 29 year old had 2 children each and 1 elective termination of pregnancy. All of them remained protected from becoming pregnant over 26-32 cycles, at top edge represent the menstrual events which remained regular, solid lines denote the period over which they were exposed to pregnancy. Arrows indicate the day on which vaccine was given. Booster injections were given to keep antibody titres above 50 ng/ml [7].
Figure 6: Anti-hCG response to the HSD vaccine in 4 sexually active women of proven fertility. MRG 30 year old and TRW 23 year old had 2 children each; HJN 32 year & SVN 29 year old had 2 children each and 1 elective termination of pregnancy. All of them remained protected from becoming pregnant over 26-32 cycles, at top edge represent the menstrual events which remained regular, solid lines denote the period over which they were exposed to pregnancy. Arrows indicate the day on which vaccine was given. Booster injections were given to keep antibody titres above 50 ng/ml [7].
Eight women completed 30 cycles without becoming pregnant, nine were protected over 24-29 cycles, 12 for 18-23 cycles, 15 for 12-17 cycles and 21 for 16-11 cycles. Only 1 pregnancy occurred in 1224 cycles [7]. All women kept ovulating as indicated by their luteal phase progesterone. They had normal regular menstrual cycles. The vaccine was a unique contraceptive. It did not disturb the menstrual regularity nor normal hormonal profiles. It was fully reversible and women desiring another child, conceived normally, and gave birth to progeny similar to their brothers and sisters with no detectable abnormality [8].
Safety and reversibility studies were also carried out by the International committee of Contraception Research (ICCR) of Population Council New York. Their findings reported, also endorse the full safety and reversibility of the vaccine [9]. As regards cancers, it has been tested in Myelomas with encouraging results. It may be pointed out that a variety of cancers at advanced stage no longer responding to available drugs, express hCG ectopically [2, 10]. Our studies have shown that PiPP, a high affinity antibody binding to hCG, kills A549 lung cancer cells in a dose-dependent manner (Fig. 7).
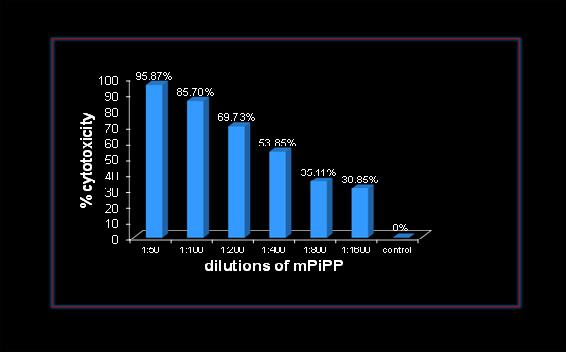 Figure 7: Dose Dependent Cytotoxicity exercised by a monoclonal anti-hCG-antibody cPiPP on A549 Lung Cancer cells [2].
Figure 7: Dose Dependent Cytotoxicity exercised by a monoclonal anti-hCG-antibody cPiPP on A549 Lung Cancer cells [2].
Similarly PiPP conjugated to Curcumin, a potent anti-cancer compound killed the Molt-4, acute lymphoblastic leukemia cells, providing rationale for use of anti-hCG antibodies for the targeted and selective immunotherapy of hCG positive cancers (Fig. 8). Thus a potent vaccine against hCG may prolong the lives of these “given-up” patients.
 Figure 8: Photomicrograph of MOLT-4 cells after incubation with cPiPP alone and cPiPP-curcumin conjugate. Cells incubated in culture medium are used as control [11].
Figure 8: Photomicrograph of MOLT-4 cells after incubation with cPiPP alone and cPiPP-curcumin conjugate. Cells incubated in culture medium are used as control [11].
Realizing the dual utility of anti-hCG vaccine for Birth Control and for Advanced Stage Terminal cancers, a recombinant version of the vaccine, hCGβ-LTB has been developed. It has been passed on to an established Company for supply of the vaccine prepared under GMP conditions. The Product manufactured by them is undergoing clinical trials in women to confirm its safety, immunogenicity, efficacy and reversibility.
I would like to pay Homage to Dr. Sheldon J. Segal, Director ICCR Population Council, Dr. Herold A. Nash, Staff Member, Population Council, Rockefeller University, New York, Dr. Elsimar M. Coutinho, Federal University of Bahia, Brazil, and Dr. Tapani Luukkainen, University of Helsinki, Helsinki, Finland, who are no more with us and without whose support and clinical trials, the vaccine against hCG would not have materialized.
- Talwar GP, Chaudhuri MK and Jayshankar R (1992). Inventors. Antigenic derivative of GnRH. UK Patent, 2228262.
- Talwar GP, Gupta Jagdish C, Kumar Yogesh, Nand Kripa N, Ahlawat Neha, Garg Himani, Rana Kannagi, Bhat Hilal (2014). Immunological approaches for treatment of advanced stage cancers invariably refractory to drugs. Journal of Clinical & Cellular Immunology, 5(4) 247.
- Talwar GP, Gupta SK, Singh V, Sahal D, Iyer KS, Singh O (1985). Bioeffective monoclonal antibody against the decapeptide gonadotropin-releasing hormone: Reacting determinant and action on ovulation and estrus suppression. Proceedings of the National Academy of Sciences, 82, 1228-1231.
- Talwar GP, Diwan M, Dawar H, Frick J, Sharma SK and Wadhwa SN (1998). Counter GnRH vaccine. In Rajalakshmi M and Griffin PD (eds) Male Contraception Present and Future. New Age International, New Delhi, 309-318.
- GP Talwar, O Singh, LV Rao (1988). An improved immunogen for anti-human chorionic gonadotropin vaccine elicited antibodies reactive with a conformation native to the hormone without cross-reaction with human follicle stimulating hormone and human thyroid stimulating hormone. Journal of Reproductive Immunology 14(3), 203-212.
- Talwar GP, Hingorani V, Kumar S, Roy S, Banerjee A, et al., (1990). Phase I clinical trials with three formulations of anti-human chorionic gonadotropin vaccine. Contraception 41(3), 301-316.
- Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, et al., (1994). A vaccine that prevents pregnancy in women. Proceedings of the National Academy of Sciences USA, 91(18), 8532-8536.
- Singh M, Das SK, Suri S, Singh O, Talwar GP (1998). Regain of fertility and normality of progeny born at below protective threshold antibody titres in women immunized with HSD-TT vaccine. American Journal of Reproductive Immunology, 39(6), 395-398.
- Nash H, Johansson ED, Talwar GP, Vasquez J, Segal S, Coutinho E, Luukkainen T, Sundaram K (1980). Observations on the antigenicity and clinical effects of a candidate antipregnancy vaccine: beta-subunit of human chorionic gonadotropin linked to tetanus toxoid. Fertility and Sterility, 34(4), 328-335.
- Talwar GP, Gupta JC, Shankar NV (2011). Immunological approaches against human chorionic gonadotropin for preventing unwanted pregnancy and therapy of advanced stage cancers expressing hCG/subunits. American Journal of Reproductive Immunology 66(1), 26-39.
- Talwar GP, Puruswani S, Vyas HK (2010). Immunological approaches against human chorionic gonadotropin for control of fertility and advanced stage cancers expressing ectopically hCG, in: Kumar A, Rao CV, Chaturvedi PK, eds. Gonadal and Nongonadal Action of Gonadotropins. Narosa Publishing House, New Delhi,183-196.













