Article / Original Article
Founder and Owner, Medicea Medica srl, Italy.
Claudio Bencini, MD, FICS, FBHS,
Founder and Owner,
Medicea Medica srl,
Italy.
13 September 2025 ; 29 September 2025
Colostomy patients face significant challenges in maintaining dignity, autonomy, and social participation due to the loss of voluntary stool control (Krouse et al., 2009). Conventional appliances provide containment but not continence (Ratliff & Scarano, 2013; Steele et al., 2019). Here we present the development of an innovative user-friendly inflatable device designed to restore partial continence in colostomized patients. Building on decades of clinical experience and prior research published in Colo-Proctology (1986) (Steele, 1986), this article reviews the anatomical rationale, technological advancements, prototype development, and potential impacts on patient quality of life and environmental sustainability.
The creation of a colostomy, often following colorectal cancer surgery, results in the permanent or temporary loss of anorectal continence. Although stoma bags provide an essential containment solution, they cannot replicate the physiological function of the anal sphincter (Shabbir & Britton, 2010; Ratliff & Scarano, 2013). Patients frequently experience social withdrawal, discomfort, and limitations in physical and intimate activities (Krouse et al., 2009). Consequently, the development of occlusion devices—colostomy plugs—has emerged as a strategy to restore control rather than mere containment (Shabbir, & Britton, 2010).
- Stoma Irrigation: provides partial control but is time-consuming and poorly accepted (Christensen & Krogh, 2010).
- Stoma Caps and Plugs: devices such as the Coloplast Alterna plug or Convatec Vitala offered temporary solutions but were discontinued or poorly adopted (Shabbir & Britton, 2010).
- Implanted Devices: including the Erlangen magnetic ring, which provided continence in 68% of cases but caused complications such as infection, stenosis, and necrosis (Williams et al., 1989).
- Historical Attempts: Lamson (US Patent 2,324,520, 1943) proposed balloon-based occlusion, but biocompatible materials and valves were unavailable at the time (Lamson, 1943).
In 1986, Bencini published in Colo-Proctology a pioneering study on the first inflatable device for colostomy continence (Bencini, 1986). Nine patients (7 men, 2 women) were enrolled in clinical trials. The device was worn during daytime hours, while a conventional colostomy bag was used at night. The follow-up lasted up to three months.
Results showed significant improvements in patients’ social life and no major inconveniences during use (Krouse et al., 2009). However, limitations emerged: the spigot and external disc were too rigid, impairing body movements and reducing comfort. These findings demonstrated both the feasibility of inflatable continence devices and the need for softer, more advanced biomaterials.
A novel inflatable continence device (Fig.1), was designed to overcome these limitations. Its structure integrates a softer spigot and a flexible external disc, aiming to improve comfort and adaptability to abdominal movements, while preserving continence and minimizing tissue trauma.
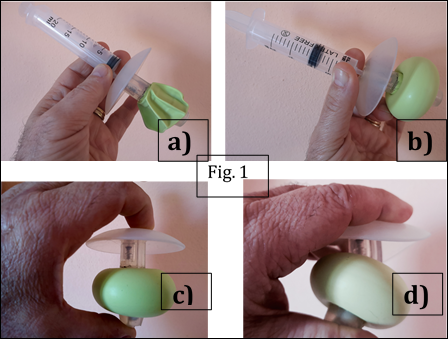
The fundamental anatomical insight was that inflating a balloon within the rigid stoma tunnel causes ischemic compression of the bowel mucosa. By contrast, inflating the balloon beyond the abdominal wall, in a compliant environment, allows safe lateral expansion and the formation of a toroidal seal (Fig.2). This principle transforms the balloon into a functional anti-reflux valve, restoring continence while respecting anatomy (Fig.3).
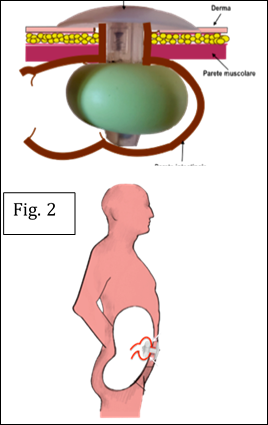
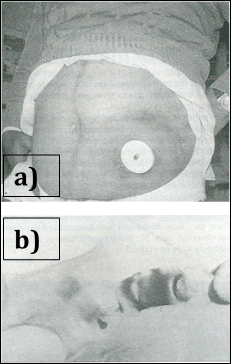 Figure 3: a) 1986 stiff device in place b) Barium enema showing valve effect
Figure 3: a) 1986 stiff device in place b) Barium enema showing valve effect
Advances in medical-grade polymers and manufacturing processes have enabled the transition from concept to viable prototype:
- Materials: Liquid Silicone Rubber (LSR) injection molding and dip-molded elastomeric balloons.
- Design:one-piece body and spigot, inflatable toroidal balloon, integrated valve.
- Production: CAD-assisted 3D design, precision tolerances, clean-room automated molding.
- Function: device inserted deflated, then inflated beyond the abdominal wall; balloon pressure kept under 30 cm H₂O ensuring safety and comfort.
The new device (StomAid®) is designed to provide:
- Quality of Life improvements: enabling sports, travel, intimate life, and social reintegration (Krouse et al., 2009; Marquis et al., 2005).
- Health benefits: preventing peristomal skin irritation and stenosis, reducing psychological burden (Shabbir& Britton, 2010; Ratliff & Scarano, 2013).
- Ease of use: simple insertion and removal by the patient.
- Safety: non-ischemic balloon expansion, no implanted metal parts, and reversible continence.
Compared to stoma bags, the device generates only 4.5% of the waste volume (Bencini, 2021). As it is washable and reusable, it avoids the continuous disposal of feces-filled plastic pouches, reducing ecological footprint and healthcare system costs.
From the early attempts in 1986 to today’s advanced prototypes, the pursuit of colostomy continence has shifted from constraint to continuity. By combining anatomical logic with modern biomaterials, the inflatable toroidal balloon device offers a safe, patient-controlled, and environmentally sustainable solution. Clinical trials are warranted to validate long-term efficacy and safety, but preliminary evidence suggests a transformative potential for millions of colostomized patients worldwide.
The author, Dr. Claudio Bencini, is the Founder and Owner of Medicea Medica srl, the company that is developing and producing the StomAid device. This potential conflict of interest is disclosed in accordance with good scientific practice. The author declares that the research and reporting have been conducted in good faith, with the intention of contributing to scientific progress and patient welfare.
- Krouse, R. S et al., (2009). Quality of life outcomes in patients with colostomy. Dis Colon Rectum, 52(2), 239–249.
- Ratliff, C. R., & Scarano, K. A. (2013). Ostomy appliance and skin care management. J Wound Ostomy Continence Nurs, 40(5), 489–495.
- Steele, S. R et al., (2019). Practice parameters for ostomy surgery. Dis Colon Rectum, 62(2), 156–179.
- Bencini C. (1986). Achieving Colostomy Continence by a New Inflatable Device. Colo-Proctology, 8(3), 170–173. https://www.researchgate.net/publication/301635659_Achieving_Colostomy_Continence_by_a_New_Inflatable_Device
- Shabbir, J., & Britton, D. C. (2010). Stoma complications: a literature overview. Colorectal Dis, 12(10), 958–964. DOI: https://doi.org/10.1111/j.1463-1318.2009.02006.x
- Christensen, P., & Krogh, K. (2010). Colostomy irrigation for prevention and treatment of stomal complications. Tech Coloproctol, 14(4), 307–311.
- Williams, J. G, et al. (1989). The Erlangen magnetic ring for colostomy continence: clinical results and complications. Br J Surg, 76(7), 692–695.
- Lamson, O. F. (1943). Apparatus and Method for Closing Abnormal Openings in Wall-like Members of the Anatomy. US Patent. 2,324,520. https://patents.google.com/patent/US3505988A/en
- Marquis, P et al., (2005). Development and validation of the Stoma-QOL questionnaire. Dis Colon Rectum, 48(9), 1629–1636.
- Bencini, C. (2021). Prosthesis for Enterostomized Patients. US Patent US2018353319A1. https://patents.google.com/patent/US20180353319A1/en













