Article / Case Report
1BronxCare Hospital Center, Department of Internal Medicine, Bronx, NY, USA
2BronxCare Hospital Center, Division of Cardiology, Bronx, NY, USA
Elina Shrestha, MD
PGY-1, Department of Internal Medicine
Bronxcare Health System
Icahn School of Medicine
1650 Grand Concourse
Bronx
NY 10457
30 April 2021 ; 17 May 2021
COVID-19 is associated with elevated inflammatory markers and a hypercoagulability state leading to venous thromboembolism. We present an atypical presentation of a young female with Internal Jugular Vein (IJV) thrombosis, a cause attributable to the disease.
A 47-year-old female with a past medical history of diabetes mellitus, hypertension, bronchial asthma, morbid obesity, uterine cancer and iron deficiency anemia (IDA) presented to the hospital with neck swelling and pain on the right side for the last week. The patient had a low-grade fever of 100.3 degrees F at home with neck discomfort, productive cough and sore throat. She was seen in the Emergency Department about 1 week back for cough and was discharged.
A computerized tomography (CT) scan of the neck and soft tissue with contrast showed the right IJV almost entirely thrombosed from its origin at the skull base to the right subclavian vein and mild pulmonary edema suspected by diffuse ground glass opacity within the lungs.
The patient was receiving intravenous iron therapy for IDA through a porta-catheter, placed 7 months ago. The right central vein subcutaneous porta-catheter access was removed, begun on systemic anticoagulation with Warfarin. SARS-CoV-2 RT-PCR was not done in this visit. On follow up, Anti-SARS-CoV-2 antibodies were positive. A repeat CT scan of the neck after 6 months of anticoagulation was done, and the thrombus was resolved.
We would like to emphasize more about the hypercoagulability in SARS-CoV-2 patients and broaden our vision for all possibilities of thrombosis, like the IJV thrombosis in our patient. We recommend a full hypercoagulability workup and imaging for prompt diagnosis and management of thromboembolic events in SARS-CoV-2 patients. There should be a low threshold for the SARS-CoV-2 PCR test and a vigilant attitude while we discover more of the disease.
COVID-19; Thrombosis
CT : Computerized Tomography
IDA : Iron Deficiency Anemia
IJV : Internal Jugular Vein
IV : Intravenous
RT-PCR : Reverse Transcription Polymerase Chain Reaction
SARS : Severe Acute Respiratory Syndrome
SARS-CoV-2 : Severe Acute Respiratory Syndrome Coronavirus-2
The coronavirus disease pandemic has posed new challenges in the daily lifestyle worldwide, especially so to the physicians with its varied presentation. The new genera of coronavirus seen as an outbreak in December 2019 with human infection is the Severe Acute Respiratory Syndrome (SARS) coronavirus 2 (SARS-CoV-2) named as COVID-19 by the World Health Organization. There are around 142 million confirmed cases of COVID-19 and around 3 million deaths due to the disease to date [1]. The more common signs and symptoms are fever (83%–98%), cough (76%–82%), and shortness of breath (31%–55%) [2]. with the disease being relatively new, different amusing ways of its presentation are being discovered, especially in the younger population. The disease is associated with elevated inflammatory markers and a hypercoagulability state leading to venous thromboembolism. We present an atypical presentation of a young female with Internal Jugular Vein (IJV) thrombosis, a cause attributable to COVID-19.
A 47-year-old female with a past medical history of non-insulin-dependent diabetes mellitus, hypertension, bronchial asthma, morbid obesity, uterine cancer and iron deficiency anemia (IDA) presented to the hospital with neck swelling and pain on the right side for the last week. The patient had a low-grade fever of 100.3 degrees F at home with neck discomfort, productive cough and sore throat. She was seen in the Emergency Department about 1 week back for cough and was discharged with Prednisone and Azithromycin for 5 days. The patient had a sleeve gastrectomy done 2 years back, family history was significant for colon cancer in father, brother and uterine cancer in mother. Social history was not significant for smoking, alcohol or any recreational drug use.
Her vital signs on presentation included a body temperature of 99.3 degrees F, BP of 139/77 mm Hg, sinus tachycardia of 114 beats/min, respiratory rate of 17 breaths/min, and oxygen saturation of 98% in room air. Her white blood cell count was increased, with low hemoglobin and normal platelets. The basic metabolic panel was within the normal range (Table 1).
| RBC | 3.64 |
|---|---|
| WBC count | 11.8 |
| Neutrophil % | 70.4 |
| Platelet | 362 |
| Hemoglobin | 10.2 |
| Hematocrit | 32.1 |
| PT | 14.8 |
| INR | 1.25 |
| D-Dimer | 1253 |
| Factor VIII | 274 |
| Partial Thromboplastin Time (APTT) | 83.2 |
| Sodium | 139 |
| Potassium | 3.7 |
| Chloride | 99 |
| CO2 | 25 |
| Glucose | 77 |
| Blood Urea Nitrogen | 6 |
| Creatinine | 0.8 |
| Calcium, Total | 9 |
| Iron | 20 |
| Ferritin | 105 |
| Unsaturated Iron Binding Capacity | 182 |
Table 1: Pertinent laboratory results
A computerized tomography (CT) scan of the neck and soft tissue with contrast showed the right IJV almost entirely thrombosed (Figure 1) from its origin at the skull base to the right subclavian vein and mild pulmonary edema suspected by diffuse ground glass opacity within the lungs (Figure 2).
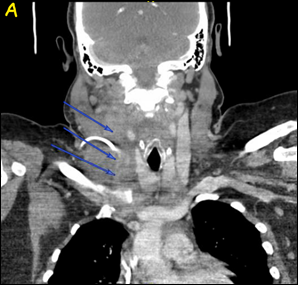
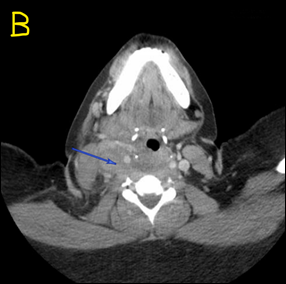
Figure 1: CT scan of the neck and soft tissue with contrast showed right IJV almost completely thrombosed, marked by the blue arrows, in Coronal view (Figure 1A) and Axial view (Figure 1B).
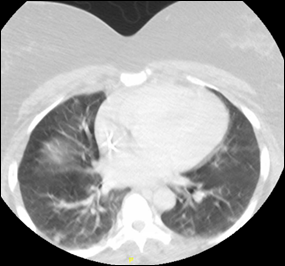 Figure 2: CT showing diffuse ground glass opacity within the lungs.
Figure 2: CT showing diffuse ground glass opacity within the lungs.
Further questioning revealed that the patient was receiving Intravenous (IV) iron therapy for IDA through a porta-catheter, placed 7 months ago. The right central vein subcutaneous porta-catheter access was removed, and she was begun on systemic anticoagulation with Warfarin. Additional workup showed high factor VIII, D-dimer, low serum iron, and negative blood culture (Table 1). SARS-CoV-2 RT-PCR was not done in this visit. On follow up, retrospective thinking raised the suspicion of COVID-19 as the cause of IJ thrombus. Anti-SARS-CoV-2 antibodies were sent which came back positive supporting our speculation. A repeat CT scan of the neck after 6 months of anticoagulation was done, and the thrombus was resolved.
COVID-19 is associated with a pro-inflammatory and hypercoagulability state. The exact mechanisms for the pathophysiology are yet under investigation. Thrombotic events are thought to result from the direct effect of COVID-19 through angiotensin converting enzyme-2 and the indirect inflammatory effects of cytokines activating vascular endothelial cells and causing endothelial injury with resultant prothrombotic properties [3].
Mackman et al in their review article collected the incidence of thrombosis in COVID 19 in six different countries, China, France, Italy, Spain, The Netherlands and The United States; they reported venous thromboembolism rates from 0.9 % to 6.5 % for non-critically ill hospitalized patients, and the rates of arterial thrombotic events between 2.8 to 3.8 % [4].
Han et al performed coagulation studies in 94 patients with confirmed SARS-CoV-2 infection and compared with 40 healthy controls. They found that antithrombin values in patients were lower and D-Dimer, Fibrin/FDP and fibrinogen were substantially higher than the control group [5]. In a study by Fu et al, they report acute ischemic stroke in two middle aged patients occurring one week after the SARS COVID-19 infection. The lab findings were thrombocytopenia, elevated cytokines and D- Dimers with the diagnosis of ischemic stroke supported by head CT and head/neck arterial CT Angiogram findings of small vessel occlusion [6]. Our patient had high factor VIII and D- Dimer, with the confirmation of thrombosis through CT scan of the neck.
Thrombus formation in general is a major cause of failure of the blood contacting medical devices like IV catheters [7]. The presence of port a catheter in our patient already puts her at risk for the occurrence of thrombus, but the fact that there were no significant events of thrombosis for the last 7 months of catheter insertion, led us to search for other possible contributing factors.
There are several cases reported supporting an increased tendency for thrombosis in regards to COVID-19 infection. Lacour et al reported a case of 68 yrs. old man with STEMI and asymptomatic COVID-19 who underwent coronary angioplasty and developed 2 episodes of acute stent thrombosis despite optimal medical therapy [8]. Similarly, Vailati et al reported a case of 62 yrs. old COVID-19 positive patient with midline and continuous positive airway pressure helmet who developed axillary vein thrombosis. They recommend use of shorter midlines and practice of prophylactic therapy with Low Molecular Weight Heparin to decrease the risk of thrombosis in COVID-19 [9]. Both of these cases discuss about the possibility of increase in thrombosis with COVID-19 infection.
There was no typical hospitalization related to COVID-19 infection in our patient but her CT chest finding of ground glass opacities with a history of treated fever and cough, along with the positive anti SARS antibody later, supports SARS-CoV-2 infection. We believe that COVID-19 related hypercoagulable state caused the development of IJV thrombus.
The management of thrombosis in COVID-19 is largely similar to non-COVID; the selection of drugs is individualized according to the patient’s specific condition with attention to hemorrhages, hepatic and renal function and several drug interactions. The specific recommendations for the prevention of thrombosis related events are yet to be made after additional studies [10]. Our patient was treated with warfarin and there was a good response with resolution of thrombus on follow up imaging.
COVID –19 has posed our generation of physicians with the challenge of recognizing the disease in its masquerading ways of presentation. Sharing the different versions of its unique presentations is the scientific way of getting victory over it. Thus, we would like to emphasize more about the hypercoagulability in SARS-CoV-2 patients and that be not limited to Deep Vein Thrombosis or Pulmonary Embolism and broaden our vision for other possibilities of thrombosis, like the IJV thrombosis in our patient. We recommend a full hypercoagulability workup and imaging for prompt diagnosis and management of thromboembolic events in SARS-CoV-2 patients. Also, there should be a low threshold for the SARS COVID RT-PCR test and a vigilant attitude while we discover more of the disease.
Grant Support: None
Disclosures: None of the authors report any conflict of interest
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. n.d. Available from: https://covid19.who.int/ (Accessed 4 April 2021)
- Wu YC, Chen CS, Chan YJ (2020). The outbreak of COVID-19: An overview. J Chin Med Assoc, 83(3), 217-220. doi: 10.1097/JCMA.0000000000000270. PMID: 32134861; PMCID: PMC7153464.
- Gómez-Mesa JE, Galindo-Coral S, Montes MC, Muñoz Martin AJ (2021). Thrombosis and Coagulopathy in COVID-19. Curr Probl Cardiol, 46(3), 100742. doi: 10.1016/j.cpcardiol.2020.100742. Epub 2020 Nov 2. PMID: 33243440; PMCID: PMC7605852.
- Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS (2020). Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arterioscler Thromb Vasc Biol, 40(9), 2033-2044. doi: 10.1161/ATVBAHA.120.314514. Epub 2020 Jul 13. PMID: 32657623; PMCID: PMC7447001.
- Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med, 58(7), 1116-1120. doi: 10.1515/cclm-2020-0188. PMID: 32172226.
- Fu B, Chen Y, Li P. The 2019 novel coronavirus disease with secondary ischemic stroke: Two cases report [published online April 2, 2020]. BMC Neurol. doi:10.21203/rs.3.rs-20943/v1 [CrossRef] [Google Scholar] [Ref list]
- Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI (2015). Medical device-induced thrombosis: What causes it and how can we prevent it? J Thromb Haemost, 13(Suppl 1), S72-S81. doi: 10.1111/jth.12961. PMID: 26149053.
- Lacour T, Semaan C, Genet T, Ivanes F (2021). Insights for increased risk of failed fibrinolytic therapy and stent thrombosis associated with COVID-19 in ST-segment elevation myocardial infarction patients. Catheter Cardiovasc Interv, 97(2), E241-E243. doi: 10.1002/ccd.28948. Epub 2020 Apr 30. PMID: 32352633; PMCID: PMC7267248.
- Vailati D, Fusco T, Canelli A, Chiariello C, Zerla P (2020). Axillary vein thrombosis in COVID positive patient with midline and continuous positive airway pressure Helmet. J Vasc Access. 2020 Jul 15, 1129729820943424. doi: 10.1177/1129729820943424. Epub ahead of print. PMID: 32669027.
- Hashemi A, Madhavan MV, Bikdeli B (2020). Pharmacotherapy for Prevention and Management of Thrombosis in COVID-19. Semin Thromb Hemost, 46(7), 789-795. doi: 10.1055/s-0040-1714273. Epub 2020 Aug 20. PMID: 32820478; PMCID: PMC7645828.













