Article / Research
1MX Biotech Ltd., Jerusalem, 95744, Israel.
2Institute of Chemistry, Hebrew University Jerusalem, Israel.
Gerard Marx , MX Biotech Ltd, Jerusalem, 95744, Israel Tel: +972 52 521 4660
9 June 2020 ; 5 July 2020
Is “mind” universal to all neural creatures or is it unique to homo sapien, whose talent for language greatly enlarges his/her ability to recall and enunciate past experience. Philosophers have wrestled with the concept of “mind” but have not delineated whether it emanates from body or spirit. Physicists have called on quantum mechanics to provide an explanatory rationale for mental states. Unfortunately, one cannot employ the metrics of physics to formulate emotions. Computer scientists aspire to emulate the workings of the brain with binary coded algorithms. Though capable of programing a memory function in robots, they too have been hampered by an inability to encode emotions. Upon consideration, “emotions” and “memory” must be integral to the cognitive process implied by “mind”. We biochemists review two proposed processes for the formation and recall of memory. The popular neurological concept is based on “synaptic plasticity”, the ability of neurons to scupt their shape and thereby modulate their signaling functions. It suggests that morphologic and functional modifications of the synapse follow a learning experience, recalled as memory.
An alternate biochemical tripartite mechanism is based on interactions of neurons with their surrounding extracellular matrix (nECM) and dopants (metal cations and neurotransmitters (NTs)). Such a chemodynamic process seems physiologically credible in that it involves materials available to the neuron. It invokes a chemical code comprising metal-centered complexes representing cognitive units of information (cuinfo); with emotive states elicited and encoded by neurotransmitters (NTs). The neural chemical code, which evolved from primitive signaling modes of bacteria and slime mold, retained the identical signaling molecules, though augmented with additional neuropeptides. The evolved neurons became organized into ever more complex neural nets instigated a new dimension (phase) of metabolic energy, a mental state characterized by emotive memory, manifest in homo sapien as language and “mind”.
Keywords : Neurotransmitters, Trace Metals, Non-synaptic Signaling, Emotions, Neural Extracellular Matrix
Background
“Mind is ever the ruler of the Universe”.
Plato
Ever since Descartes (and even before), the mind/body problem has confounded philosophers of “mind” (Descartes, Spinoza, Bergson, Husserl, Mill. Ryle, Chomsky), to name only a few [1-10], as well as scientists of cognition [11-14].
In order to focus our discussion, we define certain terms, namely “memory”, “mind” and “consciousness” as follows:
- Feelings are sensorial perceptions.
- Emotions are recalled feelings.
- Memory- for the computer relates to a physical (chemical) code expressed as metal dopants dispersed in an inorganic silicon matrix, exemplified by memory chips [15]. These are totally without affective quality (i.e. “demotive”)[16].
- Memory for neural nets relates to the recall of experience flush with emotive overtones (i.e. emotive).
- Consciousness- pertains to the ability of all neural organisms to sense their environment and to modulate their responses thereto.
- Mind- relates to the self-reflective aspects of consciousness expressed linguistically (in homo sapien).
To comprehend consciousness and mind, one needs a rational theory of neural memory. But a difficulty in discussing this has been to describe how an affective mental state can be achieved and remembered by cells employing inanimate materials. This has been a major impediment to consider encoding emotive machines [17-27].
The neural net model first described by the histologist Cajal posited that memory resulted from the cumulative performance of sets of synaptically connected neurons [11]. Hebb, a later generation physiologist, suggested that memory was represented by the joint activation of (sparse) groups of synaptically connected neurons [12] reviewed by [28,29,30]. “Learning” and “memory” followed the strengthening (increased functionality) of synaptic connections, termed “synaptic plasticity (SP)” or “long term potentiation (LTP)”.
Modern philosophers of “mind” have attempted to address the enigmas of consciousness and memory but have been hampered by their inability to address the neuron’s signaling modalities [3,7]. For example, they present neurons as “naked”, as if they were suspended in empty space [11,31]. But in fact, neurons, including glial cells [32], are surrounded by a hydrated extracellular matrix (nECM), a structural filligree through which both electric and chemical signals pass [33,34]. Serious reservations were raised about the SP model of neural signaling [22,35-39]. In particular, it was noted that there are many non-synaptic signaling pathways (ephaptic signals) through the ubiquitous nECM around all neurons. It is an established fact that most dendrites do not make synaptic contacts with neighboring neurons [40,41,42], but peter out into nECM. Notwithstanding, most modern neurobiologists follow Hebb and attempt to model learning and memory with changes of synapse contacts and function [14,30].
The late Marvin Minsky referred to the brain as a “meat machine” [43]. Indeed, it is “meat” like other tissues, powered by metabolism, its activities circumscribed by the strictures of physiology and rules of biochemistry. But it certainly is not a “machine”. It is not manufactured nor does it convert linear force into circular movement, or magnetic force into electric current or light. Rather, the brain is an evolved organ that instigates a phase change of metabolic energy to achieve a new subjective dimension, termed ”mentality”, linguistically expressed as “mind” [5,44]. One cannot employ the metrics of physics, or the formulae of mathematics or the algorithms of computers to describe mentation [18,23,26,31,45]; citing only a few). One must adopt a new paradigm, as will be elaborated on below.
Some have proposed a material basis for the physical memory trace, the “engram” originally proposed by Semon (1911) and explored by modern neuroscientists [28](reviewed by Bruce, 2001. Lashley, a physiologist, spent 30 years trying to find the location of the engram. He concluded that memory was dispersed throughout the brain (reviewed in Jeffress, 1951).
More recent neuroscientists suggested that memory resulted from a specific type of “mnemogenic” reaction [46]. Here, learning invokes an initiator mechanism causing the activation of species X, the conversion of a molecule of X into the X ̇ form. Molecular systems appropriate for such invoked calcium/calmodulin-dependent protein kinase II (CaMKII). Other candidate reactions involved proteolysis; activated trypsin protease to cleave its inactive zymogen.
A third category of discussed mnemogenic reactions was based on the synthesis of the species involved. The classic example of this type of reaction is autoregulation of the bacteriophage, repressor which prevents transcription of the genes required for lysis, where the “mnemogenic” reaction, of a pool of amino acid builds blocks necessary to synthesize the repressor [46].
Others forwarded a transcendental approach suggesting that memory and mind may “exist independent of the physical body” [47,48]. Some philosophers debunked “spirits”, “souls” and “ghosts” [3]. Others offered quantum mechanical rationales [49,50], though they could not source emotive states.
A possible direction for producing a robot engineered consciousness was termed “operational architectonics” (OA), a framework for “brain-mind” modeling [27,51]. Unfortunately, it did not meet the conceptual challenge of resolving the issue of subjective experience i.e. emotive states. Without describing how emotive states are achieved and remembered, OA could not provide a blueprint or code for a “genuinely conscious machine”.
Speculative psychology has attempted to address the question of how memory and “mind” emerge from the brain, by focusing on mathematics or language [5,17,19,44]. The classic psychoanalytic approach to memory and mind is incomplete as it is based on language which pertains only to homo sapiens [52]. The mental processes of all neural creatures are basically emotive. Behavior and the memories of experiences are infused with emotions. The enigma of neuroscience is the mystery of how emotive mental states are achieved or encoded in memory. While it is reasonable to suppose a representational system (i.e. code) for memory as the basis for “mind”, such a code must be plausible from the perspective of evolutionary neurobiology and neurochemistry [53,54].
Cognitive scientists attempting to describe “mentality” face near insurmountable difficulties [29]. There are no objective means for technically describing the subjective aspects of mentality. The computer model crumbles under the weight of physiologic considerations. Its binary-coded “Information Theory” does not apply to mental states as it cannot describe the physiologic basis of emotions or how they are encoded.
Psychoanalysis attempts to employ verbalization, as between a patient and an analyst [52]. Though memories are rediscovered, the insights gathered in this manner do not reveal the physiologic processes underlying their manifestation. Linguistic or psychoanalytic approaches to memory lack credibility in that they are not universal to all neural creatures, but limited to homo sapien [5,52]. In common parlance, a “code” is a replacement of one letter, word or phrase for another; it is essentially linguistic [55]. But how could one encode an experiential state for memory that also applies to animals?
Following the opinion of [56].
Chemistry is the only physical science which offers a pathway to understanding animate biology.”
we have embarked on a quest to identify the molecular underpinnings of the neural memory code underlying “mind”.
We take guidance from the Darwinian concept: The brain evolved from simpler aggregates of cells [57]. It must operate under the same biochemical constraints as all other tissues. But what models for a memory code are appropriate?
One must recognize that “mind” reflects psychic states (emotions) which are inextricably linked (entangled) to physiologic sensations (feelings) and reaction (emotions). These all are simulaneously instigated by neurotransmitters , as summarized in Table 1.
| Neurotransmitter (NT) | Physiologic effects * (feelings, sensations) |
Psychic states! (emotions, moods) |
|---|---|---|
| Biogenic amines (8) Amino acids (>10) Neuropeptides (>70) Acetylcholine (1) NO (1) Endocannabinoids (>10) (trace metals; >10) |
Breathing Blinking Blood pressure Blood coagulation Cold (feel) Contraction of muscles Coughing Cramps Crying Defecation Dilation of muscles Dilation of pupil Drooling Erection Evacuation Fever Goose bumps Heart beat Heat (feel) Hunger (feel) Immune reactions Itching Pain Panic Retching Seeing Shivering Smelling (feel) Thirst (feel) Touching (feel) Vomiting |
Anxiety Aggression Awareness Craving Curiosity Depression Desire Disgust Dread Dreams Fantasy Fear Hate Joy Love Lust Paranoia Sadness Sex drive Sociability |
| * No memory required. | ! Require memory. |
Table 1: Neurotransmitters (NTs), which link physiologic reactions with psychic states.
Biogenic amines, amino acids, acetylcholine and NO are all employed by bacteria a signaling molecules [58]. The neuropeptides were later developed by the evolving neural net to serve as additional signaling molecules, all included in the term “neurotransmitters” (NTs), which elicit physiologic reactions termed “feelings” as well as emotive (mental) states. One could consider “emotions” as remembered “feelings”. Table 1 makes it clear that mental states (i.e. emotions) are inextricably linked (entangled) with physiologic responses instigated by NTs.
Currently, the best that the clinical cognitive scientist can do is correlate electrodynamic signals (EEG) with neural activity or non-invasively monitor the metabolic activity of certain regions of the brain (fMRI, PET). For example, an EEG technique noted that dynamic high frequency oscillatory coupling (called “ripples”) appeared to enable different brain regions to cooperate in processing cognitive information to generate memory [59]. In spite of numerous allusions to a “code” or “syntax” for such “ripples”, no such have been identified in the true meaning of these terms. They merely buttress the idea that different anatomic compartments of the brain are metabolically coupled during the generation of mental activity. Such techniques do not resolve the core enigmas of mental processes and memory, for which there are no manipulative tools, save recreational and therapeutic chemicals (drugs) (Table 2).
| Legal | Illegal |
|---|---|
| Alcohol | Cannabis |
| Barbiturates | Cocaine |
| Caffeine | Heroin |
| Prozac* | Ecstacy |
| Ritalin* | LSD |
| Lithium salt | Smack |
| Sedatives* | Many others |
*Require medical prescription
Defining “Memory”
“Memory” can be variably defined:
- For computers, it refers to the algorithmic processing of stored bits of “information”; the retrieving of bits of information stored as dopants in a matrix for algorithmic processing [15,19,23,26,31,44,45].
- For metallurgy, a shape of a memory alloy can be deformed; when heated (i.e. above 500oC) , it “remembers” its original shape and returns to its pre-deformed shape (Wilkes, 2000).
- Immunologists use the term “memory” to describe developmental biology. For example, a lymphocyte exhibits functional plasticity by differentiating morphologically and functionally from a quiescent, long-lived “memory state” into an “effective state”, characterized by short-lived recognition of antigens and cytotoxicity toward cancer cells (Henning et al, 2018). But this is not a conscious process or involve mental states.
- For neural creatures, “memory” refers to the recall of previous experience (i.e. learning, remembering). It is inherently post-genetic and cannot be genetically transmitted. In particular, the recall of a previous experience with emotive overtones is especially potent.
Non-invasive MRI techniques have been used to monitor the plasticity of the hippocampus and neo-cortex resulting in learning [60]. Though diffusion-weighted MRI could detect changes in brain structure which correlated metabolism with learning, the structural and temporal resolution of this technique left much to be desired. For example, the size of the monitored area was on the order of 1 cm2. Also, it took a few hours for changes to be noted. At that scale and with brain volume of 1 liter, there does not seem to be enough rooms to encode and store a lifetime of experiences in memory. Moreover, one can expect that memory (engram) formation should be faster, on the order of 0.1 sec or less.
Implanted electrodes (rats), have been used to clarify the role of the hippocampus on episodic (subjective) memory and spatial recognition [59,61]. Firing rate “maps” from the activity of single neurons from the lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC), revealed major inputs from these regions to the hippocampus. However, electrodynamic firing rates in one direction or another do not reveal the underlying memory process; firing cannot function as repositories for either affective or spatial past experience.
Homo sapiens have developed tools which can mimic and measure all manner of external stimuli (i.e light, sound, weight, smell, etc) to which the neural sensing system is sensitive. But tools cannot analytically describe mental states, referred to as “emotions” or “moods”. Neither can they describe the experiential state accompanying a sensation (i.e. the color red or blue, the smell of mint, orgasm, etc). At best, these can be correlated to metabolic activity (by fMRI) or modulated by drugs like anti-depressants or “uppers” or “downers” (Table 2), by mechanisms that are not quite understood, though their chemical basis cannot be denied [62].
Emotions and Memory
Neural memory is central to all cognitive processes described by words like “thought”, “emotions” and “mentation”, resulting in “mind”.
“Memory” seems more accessible to analytic comprehension than “mind”, particularly as it is the core function of computers for which there exists a mature technology involving the fabrication of “memory materials” used to construct “memory chips”[16]. But neural memory is invisible and intangible; it cannot be numerically programmed, only experienced. This is a key obstacle to developing “artificial consciousness” in robots [20,24,25,27,51].
Memory, Language and Mind
Mental states can be considered to be organizational features of physical matter (i.e. the brain). Neural memory relates to the psychic experience of recall by a neural system that experiences emotive states. But that does not mean that the brain operates like a computer or that binary-coded “information” is equivalent to multinary “cognitive information” (cog-info) [63]. The former is objective, bereft of subjective content; the latter defines the affective states of neural sensibility.
“Mind” is unique to homo sapien in that it is expressed in linguistic terms, not accessible to animals. It is impossible to consider language without access to memory. Without memory, one could not construct a vocabulary to refer to a specific item or action, as well as synonyms, declentions, tenses, etc. In short, without the talent of memory, there is no language. Thus, memory is the key talent of “mind” for which we aspire to ascribe a causal mechanism.
Taxonomy of Memory
The brain, which is divided into specialized compartments, is the organ of memory. But as an anatomic description the whole brain is too gross, many neuroscientists have focused on the cellular scale. Neurons are generally presented as highly extended cells organized as densely packed bundles, but not in direct contact with one another. They are generally represented as suspended in undefined “space”. They almost touch to form networks via synaptic contacts which are involved in signaling. It has been conjectured tha memory resides in the physiology of neurons with synaptic contact [30,68]. By virtue of “recognition”, the brain retrieves information from the neurons in the cerebral cortex. But this begs the issue of how cognitive information is physically embodied by those neurons, so that it can be recalled to be acted upon.
Efforts have been expended in trying to graphically categorize a taxonomy of neural memory types [72] that summarizes the relationship of different types of memory (i.e. short term (STM), long-term (LTM), explicit, implicit, etc.). as in Figure 1.
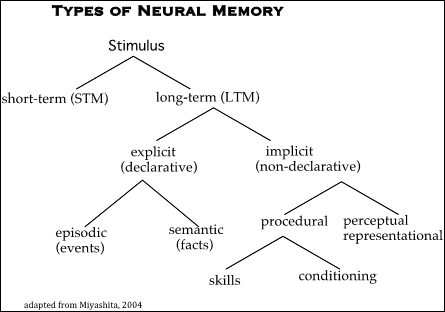
Figure 1: Taxonomy of memory (adapted from Miyashita, 2004).
Major problems with such a graphic scheme are:
- LTM must derive from STM. It cannot be a separate branch of stimulus.
- There is no mention of emotive states.
- There is no reference to physiology or to biochemical processes.
- There is overemphasis on linguistic aspects (i.e. “semantic”, “declarative”), which only apply to humans
Regarding the “semantic“ (declarative) category referred to in the above taxonomy (Figure 1), the large body of novels, plays, poems, songs and psychoanalytic descriptions of recalled emotive states do not provide an explanatory rationale. In spite of attempts by linguists and psychologists to grapple with the mystery of memory as the “source function” for other mental activities their efforts have not clarified the physiologic basis for mental processes [44,52,69].
The evolution of Life from simple beginnings to complex organisms can also be applied to memory and emotions. For example, medical treatments are based on chemical descriptions of metabolism as well as structure. Though it is not possible to describe the physiology of an emotive state algorithmically, graphically or textually, chemistry provides a molecular rationale. For the neural creature, NTs are molecules which elicit both “feelings” (physical reactions) and psychic states termed “emotions” (Table 1). Thus, chemistry provides a “window” into biology as well as Mind.
[34,63].
A suggested tripartite mechanism and chemographic representation (Figure 2) seems credible.
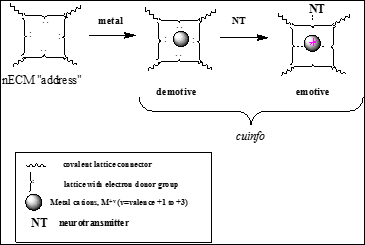
Figure 2: Chemographic representations of the reaction of a nECM (electron rich site) binding site for a metal cation, an “address”. The binding of an electron-rich neurotransmitter (NT) to the metal-centered cognitive unit of information (cuinfo) confers emotive context [39].
This mechanism is consonant with experimental observations relating to the morphology of neurons as it relates to their interactions with their surrounding matrix (nECM). It also involves materials available to neurons (i.e. trace metals, NTs). Based on the entanglement of physiologic and psychic effects, the neurotransmitters (NTs) appear to serve as the elicitors and encoders of emotive states.
The nECM also has a long history, appearing as an extracellular polysaccharide slime around primitive cells with a primitive memory function [64,65]. Clearly, the neural signaling system that involves NTs and nECM developed along Darwinian direction from unicellular entities.
Criteria or tests which can support such a proposed tripartite mechanism of memory, are:
- Morphology of neurons (Cajal et al)- extended surface, synaptic contacts.
- Presence and composition of nECM surrounding neurons (many refs since 1960).
- Presence and distribution of trace metals in the brain (as detected by AA, LS-MS, NAA).
- Effects of trace metals on mental processes– mood/memory and deficiency /toxicity [66,67].
- Influences of neurotransmitters (NTs) on both physiologic reactions and affective states (Table 1).
- Evidence of metal-NT complexes (many refs).
- Impedence electrodes: Prototype electronic devices coared with analogues of NT or synthetic polysaccharide analogues of the nECM. Such “neuromimetic” electrodes have been used to confirm the interactions of NTs and polysaccharides with cationic metals, which modulate electrodynamic properties [74-78].
In the light of this mechanism, a revized taxonomy of memory can be schematized as in Figure 3.
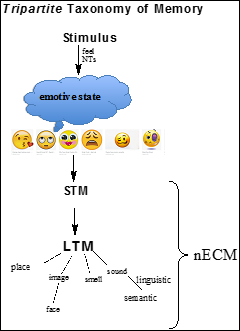
Figure 3. Schematic tripartite taxonomy of neural memory. Long term memory (LTM) is derived from short-term memory (STM). The NTs are released from neurons as a result of “feelings” (i.e. emoji) which induce multiple physiologic reactions and affective states (emotions) (Table 1). Thus, neurotransmitters (NTs) perform as instigators and encoders of psychic states, which are entangled with physiologic responses which they also elicit; the nECM performs as a “memory material”.
This taxonomic schema (Figure 3) indicates that emotive states are induced by the
originating stimulus (i.e. STM leading to LTM). Perceptive feelings directly instigate emotive states which are remembered in STM as well as in LTM.
The nECM is a glycosaminoglycan lattice around the neurons, central to the process of recall in which cognitive units of information (cuinfo) (Figure 3) are formed and stored around the neuron (Figure 4 ).
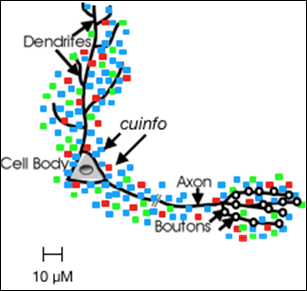
Figure 4. The neuron is surrounded by nECM (the GAG lattice not shown) which serves as a neurochemical “memory material” wherein units of encoded memories are stored as cuinfo.
The colored boxes, which represent the individual cuinfo described in Figure 2, are not to scale, as they are of molecular dimension (i.e. 10 nm) compared to the 10-100 um scale of the neuron and its parts. The different colors indicate complexes with different combinations of NTs and metal cations.
Throughout discourses in which cognition, learning and language are considered [44 Piattelli-Palmarini, 1980), tautological arguments are presented about mental processes involving language, which do not lead to an agreed-upon physiologic process. But even supposing that an “internal neural code” for mind exists, that does not reveal its structure or the rules under which it is rendered operative.
We take another tack and contemplate the evolution of emotions and memory as linked to the development chemical-signaling. Consider the energetics of bacterial signaling with the evolution of cells, nets of cells, and brains with anatomically specialized regions. One can suppose that the increasingly complex interactions of matter around the evolved neural nets instigated the emergence of a novel phase of metabolic energy, a mental state experienced as memory. The ever more complex oraganization of matter around neurons established a new phase of metabolic energy (Figure 5), a mental state manifest as “feelings , recalled as emotive memory, emerging as “mind” .
In the history of physics as a scientific discipline, the identification of “energy” as a relevant parameter, represented as a “phase change” in the conception of the physical world. So too for neuropsychology, the identification of mechanism by which neurons generate new mental dimensions represents a phase change in our conception of mind and matter, or rather, mind from matter.
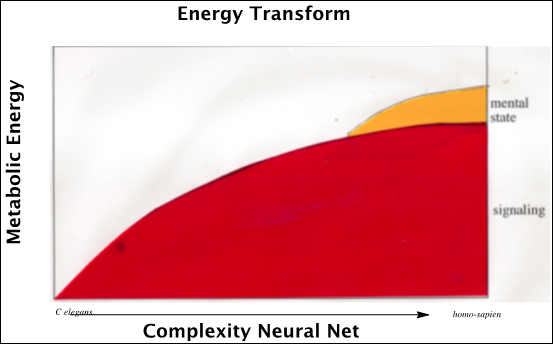
Figure 5: The evolution of neural signaling processes whereby part of the metabolic energy of ever more complex neural nets is phase changed to emerge as new mental states.
There are no tools to measure emotive states analogous to those of material scientists who test the energy and phases of matter in an objective manner [70,71]. We suggest that the evolving organization of matter around neurons permitted the emergence of a new phase of biologic energy, a mental state manifest as “feelings” recalled as emotive memory (Figure 5) [79].
We accept that psychical phenomena arose in the foundations of biology, in the tropisms of bacteria and slime molds, which employ biomodulating molecules as group signaling effectors. Such as attitude brings mental processes into the compass of natural fact. To be clear, such proceses are not revealed by philosophical musings and psychoanalytic probings a la Freud (Figure 6).
Figure 6: A schematized photograph of Freud, illustrating the types of biochemical complexes which we propose that underlay his memory and emotive states.
The tripartite mechanism of neural memory has great explanatory power, is predictive, and coherent. It interprets the persistence of memory and mind in chemodynamic terms relating to the stability of the constituent coding units (the cuinfo). Of course, such stability is somewhat flexible, witness the processes of losing one’s memory and changing one’s mind.
(By GM). In memory of my wife, the artist Georgette Batlle (1940-2009), my anchor of stability and comfort. Thanks to friends, Lilly Rivlin (New York, N.Y.) and the late Bill Needle (Eastchester, N.Y.) for their early encouragement and financial support in the period 1980-1984.
I thank my nephew JJ Marx Esq (Jerusalem) for suggesting that we include ideas for future directions. Thanks to my companion Karine Ahouva Leopold (Jerusalem, Paris) for introductions, emotional support and encouragement. I appreciate Professor Jean Paul Doguet’s (Paris) interest and suggestions regarding the relevance of the philosophers Husserl and Bergson.
CG would like to thank the Binjamin H. Birstein Chair in Chemistry.
Again, we both wish to thank Professor Gallistel (Rutgers U.) for drawing our attention to “memory” as the proper focus of our speculations.
GM is a founder of MX Biotech Ltd., with the commercial goal to develop new “memory materials”.
CG is emeritus professor of HU, but is active in developing and patenting peptide-based tools for surgery and pharmacology.
Notwithstanding, the ideas forwarded here are scientifically genuine and presented in good faith, without commercial clouding of the concepts expressed here.
- Bergson H (1908) Matter and Memory.Dover Publications, New York Translation from 1912 Edition. Allen & Co, London.
- Shearcroft WFF (1926) Matter, Man and Mind. MacMillan, NY.
- Ryle G (1949) The Concept of Mind.University of Chicago Press, Chicago, IL.
- Brough JB (1975) Husserl on memory The Monist 59: 40-52.
- Chomsky N (1975) Reflections on Language. Pantheon Books, New York.
- Lin M (2005) Memory and personal identity in Spinoza, Canadian Journal of Philosophy 35: 243-268.
- Bistricky SL (2013) Mill and mental phenomena: Critical contributions to a science of cognition.
- de Warren N (2016) Augustine and Husserl on Time and Memory.
- Simonetta D (2017) Descartes, Intuition and memory. XVII Siècle 4: 1-192.
- Toth O (2018) Memory, Recollection and Consciousness in Spinoza’s Ethics. Society and Politics 12 (2): 50-71.
- Cajal RY (1911) Cajal’s Histology of the Nervous System of Man and Vertebrates, Oxford University Press,1995 ISBN: 9780195074017
- Hebb DO (1949) The Organization of Behavior. Wiley, New York.
- Pally R (2000) Mind-Brain Relationship. Taylor & Francis Group, London.
- Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157: 163-186.
- Landauer R (1996) The physical nature of information. Physics Letters A 217: 188-193.
- Di Ventra M, Pershin YV (2011) Memory materials: a unifying description. Materialstoday 14: 584-591.
- Lotka AJ (1956) Elements of Mathematical Biology. Dover, New York.
- Fodor JA (1983) The Modularity of Mind. MIT Press, Cambridge, MA.
- Gardner H (1985) The Mind’s New Science. A History of the Cognitive Revolution. Basic Books, New York.
- Picard R (1997) Affective Computing. MIT Press, Boston.
- Churchland PM (1989) Neurophilosophy: Toward a Unified Theory of Mind. MIT Press, Cambridge, MA.
- Amsterdam Gallistel CR, King AP (2009) Memory and the Computational Brain. Wiley Blackwell, New York.
- Pickering A (2010) The Cybernetic Brain. University of Chicago Press, IL.
- Meshulam M, Winter E, Ben-Shakhar G, Aharon I (2011) Rational emotions. J. Social Neuroscience 7: 1-7. doi.org/10.1080/17470919. 2011.559124
- Hasson C (2011) Modeling of emotional mechanisms for an autonomous robot: developmental and social perspective. University of Cergy Pontoise, PhD Thesis, France.
- Guidolin D, Albertin G, Guescini M, Fuxe K, Agnati LF (2011) Central nervous system and computation. Q Rev Biol 86: 265-285.
- Fingelkurts AA, Fingelkurts AA, Neves CHF (2012) “Machine” consciousness and “artificial” thought: An operational architectonics model guided approach. Brain Research 1428: 80-92.
- Josselyn A, Kohler S, Frankland PW (2017) Heroes of the Engram. Neuroscience, 37: 4647– 4657.
- Arshavsky YI (2017) Neurons versus Networks: The interplay between individual neurons and neural networks in cognitive functions. Neuroscientist 23: 341-355.
- Asok A, Leroy F, Rayman JB, Kandel ER (2019) Molecular mechanisms of the memory trace. Trends in Neurosciences 42: 14-22.
- Sejnowski TJ, Koch C, Churchland PS (1988) Computational neuroscience. Science241: 1299-1306.
- Ashhad S, Narayanan R (2019) Stores, channels, glue, and trees: active glial and active dendritic physiology. Molecular Neurobiology 56: 2278-2299. https://doi.org/10.1007/s12035-018-1223-5
- Bandtlow CE, Zimmermann DR (2000) Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev 80: 1267-1290. doi: 10.1152/physrev.2000.80.4.1267.
- Marx G, Gilon C (2020) Interpreting neural morphology. Acta Scientific Neurology 3: March 2020.
- Amit D (2013) Hebb vs Biochemistry: the Fundamentalist Viewpoint. In selected papers by Daniel Amit (1938-2007) http://www.thefreelibrary.com/Selected+papers+of+Daniel+Amit+(1938-2007).-a0332371120
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER (2000) Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nature Reviews Neuroscience 1: 11-20.
- Arshavsky Y I (2006) The seven “sins’’ of the Hebbian synapse: Can the hypothesis of synaptic plasticity explain long-term memory consolidation?. Progress in Neurobiology 80: 99-113.
- Von Neumann J, Kurzweil R (2012) The computer and the brain.Yale University Press.
- Marx G, Gilon C (2018) The molecular basis of neural memory. Part 10. The sins and redemption of neurobiology. J Neurol Neurocrit Care Volume 1: 1-7.
- Anastassiou CA, Perin R, Markram H, Koch C (2011) Ephaptic coupling of cortical neurons. Nature Neuroscience 14: 217-223.
- Vizi ES, Fekete A, Karoly R, Mike A (2010) Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 160: 785-809.
- Vizi ES (2013) Role of high-affinity receptors and membrane transporters in non-synaptic communication and drug action in the central nervous system Pharmacol. Rev. 52: 63-89.
- Levy S (2016) Marvin Minsky’s marvelous Meat Machine. Wired, January 2016.
- Fodor JA (1975) The Language of Thought. TY Crowell Co. Inc. USA.
- Turing AM (1950) Computing Machinery and Intelligence. Mind 49: 433-460.
- Roberson ED, Sweatt JD (1999) A biochemical blueprint for Long-Term Memory. Learning & Memory 6: 381-388.
- Searle, John R (1980) Minds, brains, and programs. Behavioral and Brain Sciences 3 (3): 417-457.
- Searle JR (1997) The Mystery of Consciousness. The New York Review of Books, New York.
- Penrose R (1989) The Emperor’s New Mind.Oxford University Press, New York.
- Vannini A (2008) Quantum models of consciousness.Quantum Biosystems 2: 165-184.
- Fingelkurts AA, Fingelkurts AA, Neves CHF (2018) Mind the physics: Physics of mindComment on “Physics of mind: Experimental confirmations of theoretical predictions” by Felix Schoeller, Leonid Perlovsky, and Dmitry Arseniev Physics of Life Reviews 25: 75=77.
- Freud S (1900) The Interpretation of Dreams. Strachey, James. New York.
- Matthews GG (2001) Evolution of nervous systems. Neurobiology: molecules, cells, and systems. Wiley-Blackwell. p. 21. ISBN 978-0-632-04496-2.
- Burkhardt P, Sprecher SG (2017) Evolutionary origin of synapses and neurons – Bridging the gap. Bioessays 39: 1700024 -1700034.
- Shiffman D (2012) The Nature of Code. Magic Book Project.
- Medawar P (1967) The Art of the Soluble.Methnon Co,
- Romanes GJ (1883) Mental Evolution in Animals. With Posthumous Essay on Instinct by Charles Darwin. Kegan, Paul, Trench & Co., London. Nabu Public Domain Reprints.
- Roshchina VV (2010) Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. In: M. Lyte and P.P.E. Freestone (eds.), Microbial Endocrinology,
- Vaz AP, Inati SK, Brunel N, Zaghloul KA (2019) Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363: 975-978. doi: 10.1126/science.aau8956.
- Brodt S, Gais S, Beck J, Erb M, Scheffler K, Schönauer M (2018) Fast track to the neocortex: A memory engram in the posterior parietal cortex. Science 362: 1045-1048. doi: 10.1126/science.aau2528.
- Wang C, Chen X, Lee H, Deshmukh S, Yoganarasimha D, Savelli F, Knierim J (2018) Egocentric coding of external items in the lateral entorhinal cortex. Science 362: 945-949. doi: 10.1126/science.aau4940.
- Vytal K, Hamann S (2010) Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cognitive Neuroscience 22: 2864-2885.
- Marx G, Gilon C (2017) The molecular basis of neural memory. MBM Pt 7: Artificial intelligence (AI) versus neural intelligence (NI). AIMS Medical Science 4: 254–273. DOI: 10.3934/medsci.2017.3.25
- McCormick JJ, Blomquist JC, Rusch HP (1970) Isolation and characterization of an extracellular polysaccharide from Physarum polycephalum.J Bacteriol 104: 1110-1118.
- Reid C, Latty T, Dussutour A, Beekman M (2012) Slime mold uses an externalized spatial ‘memory’ to navigate in complex environments. Proc. U.S. Nat. Acad. Sci. 109: 17490–17494.
- Rairhall LT (1963) Industrial Toxicology. Hafner Publishing Co. London.
- Friberg IL, Nordberg GF, Vouk VB (2012) Handbook on the Toxicology of Metals. Elsevier Publishing.
- Popper KR, Eccles JC (1977) The Self and Its Brain. Part II: Eccles- neurophysiology Springer Verlag, Berlin.
- Piattelli-Palmarini M (Ed) (1980) Language and Learning:The Debate Between Jean Piaget and Noam Chomsky. Routeledge & Kegan Paul Ltd. London.
- Rice SA (2008) Advances in Chemical Physics. John Wiley & Sons. ISBN 978-0-470-23807-3.
- Wen X-G (2019) Choreographed entanglement dances: Topological states of quantum matter. Science 363: 834.
- Miyashita Y (2004) Cognitive memory: cellular and network machineries and their top-down control. Science 306(5695): 435-440.
- Lin M (2005) Memory and personal identity in Spinoza, Canadian Journal of Philosophy 35: 243-268.
- Marx G, Gilon C (2019) The tripartite mechanism as the basis for a biochemical memory engram. J. Integr. Neurosci. 18: 181-185. doi: 10.31083/j.jin.2019.02.6101.
- Maunsell JHR (1995) The brain’s visual world: Representation of visual targets in cerebral cortex. Science 270: 764-768.
- Inter-kingdom Signaling in Infectious Disease and Health, Chapter 2 Springer Science+Business Media, New York.
- Shils ME, Olson JA, Shike M (1994) Modern Nutrition in Health and Disease. Lea & Febiger, Philadelphia.
- Ungerleider LG (1995) Functional brain imaging studies of cortical mechanisms of memory. Science 270: 769-778.
- Langer S (1962) Philosophical Sketches: The Process of Feeling. Barnes & Noble; reprint edition (2009).