Article / Case Report
1St. Joseph’s Medical Center, Stockton, CA
2Central Valley Eye Medical Group, Stockton, CA
3Adjunct Clinical Associate Professor, Department of Ophthalmology, Stanford School of Medicine, Central Valley Eye Medical Group, Stockton, CA
Dr. Kimberly Cockerham, MD, FACS
Central Valley Eye Medical Group
Plastics-Orbit-Neuro
Medical appointments: 209-952-3700
Cosmetic appointments: 209-952-3705
Cell: 650-804-9270
CVEMG.com
CockerhamMD.com
7 December 2020 ; 15 February 2021
The novel Coronavirus Disease 2019 (COVID-19) is known to present with a broad range of clinical manifestations. While symptoms such as fever, cough, dyspnea, myalgias, diarrhea, anosmia, and ageusia predominate, less common manifestations involving multiple systems have also been described. Some reported ocular manifestations include symptoms associated with keratoconjunctivitis, such as chemosis, ocular pain, photophobia, dry eye and tearing [1]. Neurological symptoms in addition to smell and taste dysfunction have been commonly described as well, and include headache, ataxia, dizziness, altered level of consciousness, and stroke [2]. Whether these neuro-ophthalmologic findings reflect direct involvement of these systems or a more generalized response to SARS-CoV-2 infection remains uncertain. Many other neurologic, rheumatologic, and infectious diseases also present with similar clinical findings as those described in COVID-19, further complicating the diagnostic picture. In this case series, we examine several patients presenting with unusual neuro-ophthalmological manifestations and discuss similarities of these findings with those seen in SARS-CoV-2 infection, and review current literature describing possible mechanisms underlying similar findings in patients with confirmed COVID-19.
Keywords: COVID-19, episcleritis, giant cell arteritis, idiopathic intracranial hypertension, infectious disease, inflammatory response, neuro-ophthalmology, neurology, novel Coronavirus Disease 2019, orbital apex-sphenoid syndrome, ophthalmology, optic neuritis, pseudotumor cerebri, rheumatology, SARS-CoV-2, scleritis, Takayasu arteritis, vasculitis, vision loss
Case 1: A 33-year-old Hispanic female with a history of celiac disease and recurrent bilateral anterior scleritis and episcleritis presented with worsening pain and conjunctival injection in OD for 1 week. The patient’s previous episodes of scleritis were responsive to oral prednisone. Physical exam was significant for 1+ upper lid erythema and swelling, 2+ diffuse injection, 2+ temporal chemosis in the right eye. Fundus exam of the right eye revealed normal vitreous humor, a C/D ratio of 0.5, normal macula, vessels, periphery, and disc. OCT testing was normal in both eyes and showed normal thickness of the retinal nerve fiber layer. ESR was 6 mm/hr and CRP was 0.5. ANA, HIV, HLA-B27, RPR, Quantiferon gold, Anti-PR3 QN, and Anti-MPO QN tests were all negative. D-dimer levels were within normal limits at < 150 ng/ml. Homocysteine (12.4 μmol/L) and RF (14 IU/ml) were both borderline elevated. Measured anti-gliadin IgA antibodies were substantially elevated at 166.9 units/ml, while endomysial IgA antibodies and anti-tissue transglutaminase antibodies were negative. COVID-19 nasopharyngeal swab was negative. CT of the brain and orbits showed focal thickening and enhancement of the anterior compartment in the right globe, suggestive of anterior sclerouveitis. The patient’s symptoms remained severe despite treatment with 60 mg prednisone per day. Symptoms improved with IV methylprednisolone 250 mg every 6 hours therapy for 3 days. Following discharge, repeat CT showed marked improvement in previously observed edema and injection in the right eye. Visual acuity was 20/20 in both eyes, tonometry showed pressure of 18 mm Hg in the right eye and 14 mm Hg in the left eye. Visual fields and extraocular eye movements were full bilaterally. A diagnosis of recurrence of anterior scleritis secondary to celiac disease was made with an unknown trigger for increased severity of symptoms.
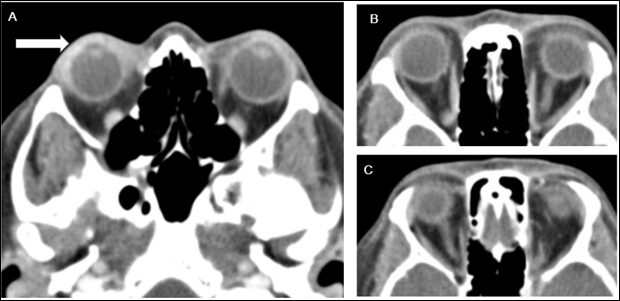
Figure 1: Computed tomography (CT) of the orbits with IV contrast of patient described in case 1. A) Axial view showing thickening and enhancement of anterior compartment of the right globe (white arrow) with associated edema, B) and C) bilateral optic nerves and extraocular muscles appear to be normal in size and caliber with mildly increased enhancement seen of bilateral medial rectus muscles.
Case 2: A 58-year-old Caucasian female with a history of insulin-dependent type 2 diabetes mellitus and hypertension pre-sented with 8 days of right upper eyelid abscess and vision loss in OD. Vision in OD was 20/400 (20/25 six months prior) and vision in OS was 20/25. Visual fields in OS were full while visual fields in OD revealed a central scotoma. Extraocular eye movements were full bilaterally. Slit lamp exam revealed erythema, edema, and 10 x 10 x 5 mm abscess on the right upper lid, and 2+ nuclear sclerosis bilaterally. Fundus examination revealed optic nerve edema with splinter hemorrhages inferiorly in the right eye. There was no presence of diabetic retinopathy. OCT examination revealed normal foveal contour and retinal nerve fiber layer thickening nasally in OD, and normal findings in OS. MRI of the brain and orbits with and without contrast was noted to be unremarkable without any evidence of mass, enhancement or other demyelinating lesions. A partially empty sella was incidentally noted. MR venography was unremarkable without any evidence of stenosis or filling defects. Lumbar puncture revealed a normal opening pressure of 5 cm H2O, and unremarkable CSF studies. Two weeks later, a repeat fundoscopic exam showed resolution of the optic nerve edema with early temporal pallor in OD. Visual acuity and visual field testing remained the same. Laboratory studies included a negative D-dimer, ANA, CRP, ESR, RPR, Quantiferon gold, and COVID-19 nasopharyngeal swab. Folate and vitamin B12 levels were normal. The patient was treated with oral clindamycin and had incision and drainage of her right upper lid abscess. Differential diagnoses included non-arteritic anterior ischemic optic neuropathy, diabetic papillitis, and optic neuropathy of infectious etiology.
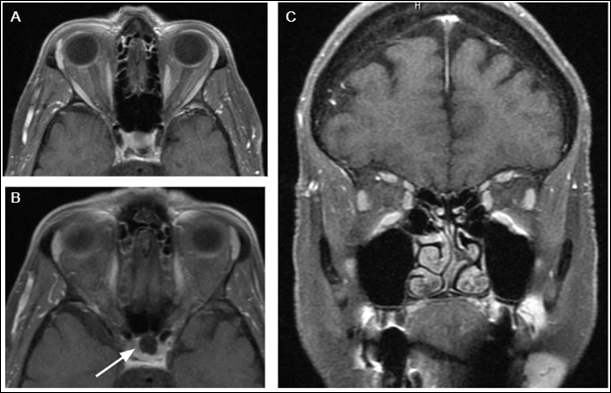
Figure 2: Magnetic resonance imaging (MRI) of the brain and orbits (all images are T1-weighted fat-saturated post-contrast) of the patient discussed in case 2. A) Axial image showing normal optic nerves and extraocular eye muscles bilaterally without abnormal thickening or enhancement. B) Axial image showing incidental discovery of partially empty sella with the majority of the pituitary region showing similar attenuation to CSF, with enhancement at the floor of the pituitary fossa. C) Coronal showing normal size and caliber of extraocular eye muscles.
Case 3: A 62-year-old Caucasian male presented with acute onset bilateral visual loss that occurred over the course of one day and subsequently stabilized. The patient reported symptoms of major depression due to the COVID-19 lockdown and heavy alcohol use of at least 4 drinks per day. No other neurologic or systemic symptoms were reported. Visual acuity testing showed 20/30 vision in OD and 20/40 in OS. Visual fields and extraocular eye movements were full bilaterally. Color vision was decreased bilaterally with 9/11 color plates correct in OD and 3/11 in OS. Funduscopic exam showed diffuse pallor and optic nerve reduced in size/caliber bilaterally. OCT tes¬ing revealed bilateral severe thinning of the retinal nerve fiber layer. A normal pituitary gland was visualized. Homocysteine was elevated at 14 umol/L. CBC, ESR, CRP, Quantiferon gold, RPR, B12, and folate were all in normal ranges. COVID-19 status and D-dimer levels were unknown. MRI of the brain and orbits showed severe bilateral optic nerve atrophy. The patient was started on prednisolone eye drops once per day. The working diagnosis was bilateral optic nerve atrophy presumably secondary to chronic excessive alcohol consumption.
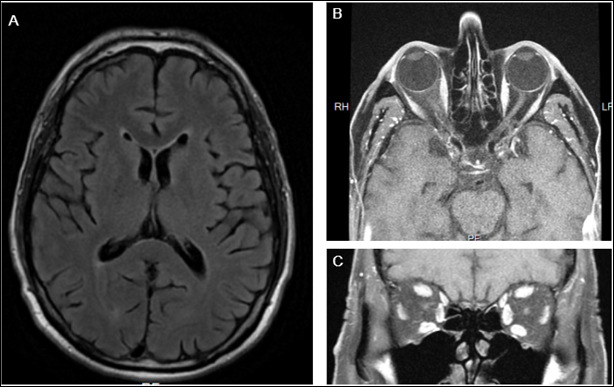 Figure 3: MRI of the brain and orbits with and without gadolinium of the patient discussed in case 3. A) Axial T2 FLAIR sequence showing nonspecific foci of hyper-attenuation B) Axial and C) Coronal T1-weighted fat-saturated post-contrast images showing atrophic optic nerves bilaterally in this patient, who reports a history of heavy alcohol use.
Figure 3: MRI of the brain and orbits with and without gadolinium of the patient discussed in case 3. A) Axial T2 FLAIR sequence showing nonspecific foci of hyper-attenuation B) Axial and C) Coronal T1-weighted fat-saturated post-contrast images showing atrophic optic nerves bilaterally in this patient, who reports a history of heavy alcohol use.
Case 4: A 73-year-old Vietnamese male with hyperlipidemia and hypertension, first complained of progressively decreased vision in 2019, especially in his left eye. At the time, it was felt to be due to mature nuclear sclerotic cataracts and he un-derwent cataract surgery in the left eye in April 2019 and was lost to follow-up. The patient reported initial improvement af-ter surgery, however his vision gradually worsened over the 6 months prior to neuro-ophthalmology evaluation in November 2020. At this evaluation, his vision in OD and OS was counting fingers at one foot (OD 20/100, OS 20/400 in April 2019), and pressures by tonometry were 18 mm Hg OD and 15 mm Hg OS. There was a relative afferent pupillary defect in his left eye which was not present in April 2019. Ishihara color testing was 0/11 for both eyes, however could identify the color red at six inches. He had a 3 plus nuclear sclerotic cataract on the right and a well centered intraocular lens on the left. Fundus examination revealed bilateral optic disc pallor (left greater than right) and adjacent clump of lipofuscin. Extraocular eye movements were full bilaterally. Humphrey Visual Field showed diffuse depression. MRI of the brain and orbits with and without contrast revealed a large 7 cm suprasellar mass compressing the optic chiasm. The anterior communicating artery and middle cerebral artery branches were distorted and compressed without occlusion. Due to the size of the mass and extent of severe vision loss, the patient was sent directly to a medical center for emergency neurosurgical intervention. He was scheduled for follow-up in four weeks for OCT and visual field examinations.
Case 5: 64-year-old Hispanic female with a history of insulin-dependent type 2 diabetes mellitus, ESRD on dialysis, and uterine cancer status post total hysterectomy presented with severe progressive bilateral visual loss progressing to complete blindness over the course of 4 months. The patient denied headache, numbness/tingling in her extremities, or any other neurological symptoms. Examination revealed severe bilateral optic disc edema and lumbar puncture revealed an opening pressure of 51 cc H2O with bland CSF profile. Visual acuity testing showed no light perception in the right eye, and light perception in the left eye. Fundoscopic examination revealed symmetrical massive optic disc edema bilaterally with peri-papillary hemorrhage that was completely confluent, forming a ring of blood around each nerve. Exudates and severe diabetic retinopathy were observed bilaterally as well. COVID status, ESR, D-dimer were unknown. CTA of the brain and noncontrast MRI of the brain revealed a possible area of attenuation unilaterally in the transverse-sigmoid venous sinus. There was no evidence of intracranial mass or hydrocephalus. Due to markedly elevated CSF opening pressure and progressive visual loss, a diagnosis of pseudotumor cerebri was suspected and the patient was surgically treated with a right ventriculoperitoneal shunt at UCSF Medical Center. Differential diagnoses included idiopathic intracranial hypertension, bilateral arteritic anterior ischemic optic neuropathy, and infiltrative process affecting bilateral optic nerves, including malignant, infectious, and inflammatory etiologies.
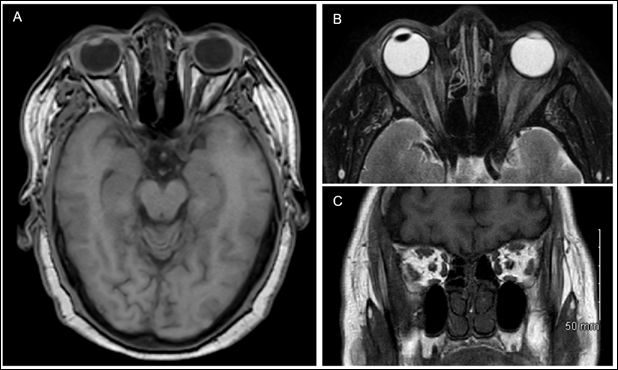 Figure 4: MRI of the brain and orbits with and without gadolinium for the patient discussed in case 5. A) Axial T1 weighted post-contrast fat saturated image unremarkable for any acute intracranial findings or evidence of hydrocephalus. B) Axial T2 weighted fat-saturated image showing evidence of left intraocular lens implant but otherwise without significant enlargement of optic nerve sheath. C) Coronal T1 weighted spin-echo image showing no enlargement of optic nerves or extraocular muscles bilaterally.
Figure 4: MRI of the brain and orbits with and without gadolinium for the patient discussed in case 5. A) Axial T1 weighted post-contrast fat saturated image unremarkable for any acute intracranial findings or evidence of hydrocephalus. B) Axial T2 weighted fat-saturated image showing evidence of left intraocular lens implant but otherwise without significant enlargement of optic nerve sheath. C) Coronal T1 weighted spin-echo image showing no enlargement of optic nerves or extraocular muscles bilaterally.
Case 6: A 29-year-old obese Caucasian female with history of seizure disorder controlled with keppra, hysterectomy due to complications from prior C-section, previously detected bilateral optic nerve edema that was presumed to be idiopathic intracranial hypertension presented with increasing bilateral orbital pain, persistent headaches, transient visual obscurations, and pulsatile tinnitus. The patient was taking Diamox 250 mg four times a day, and denied any history of prior use of steroids, lithium, OCPs, or vitamin A derivatives. The patient further denied symptoms suggestive of thyroid dysfunction. Visual testing revealed 20/20 vision bilaterally with pinhole correction, intraocular pressures of 15 mm Hg in OD and 14 mmHg in the OS, pupils that were equal, round, and reactive to light bilaterally with no APD, full visual fields, color plates, and extraocular eye movements. Fundoscopic exam revealed 360-degree edema bilaterally. ESR, CRP, ANA, COVID IgG, and Lyme Disease DNA tests were negative. Vitamin B12 levels were within normal limits and vitamin D levels were low at 20 ng/ml. Current COVID-19 status and D-dimer levels were unknown. Recent thyroid function tests were within normal limits. Prior CT scan showed mild proptosis with fat expansion but no apical compression. MRI brain and orbits showed incidental finding of partially empty sella, otherwise imaging studies were unremarkable and MR venography was negative for any findings suggestive of venous sinus thrombosis. Lumbar puncture was performed while the patient was on Diamox therapy as well which showed an opening pressure of 16 cm H2O and bland CSF studies. The working diagnosis was idio-pathic intracranial hypertension.
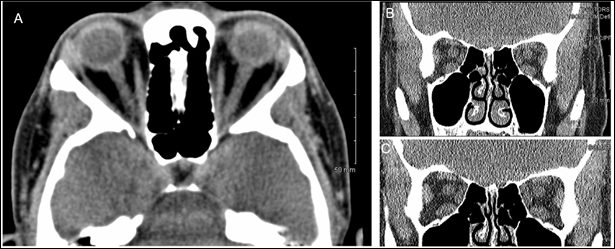 Figure 5: CT of the brain and orbits without contrast for patient discussed in case 7. A.) Axial view of CT brain and orbits without contrast showing mild right eye proptosis and incidental finding of partially empty sella. B.) and C.) Coronal views of CT brain and orbits without contrast showing slightly enlarged extraocular eye muscles but otherwise not remarkable for any other acute intracranial abnormality.
Figure 5: CT of the brain and orbits without contrast for patient discussed in case 7. A.) Axial view of CT brain and orbits without contrast showing mild right eye proptosis and incidental finding of partially empty sella. B.) and C.) Coronal views of CT brain and orbits without contrast showing slightly enlarged extraocular eye muscles but otherwise not remarkable for any other acute intracranial abnormality.
Case 7: An 86-year-old Hispanic female with a history of latent tuberculosis, hypertension, and type 2 diabetes mellitus presented with visual loss in the right eye, jaw claudication, and worsening chronic paroxysmal right-sided headache fol-lowing head trauma. Due to her positive PPD test, the patient was receiving latent TB treatment with INH and rifampin once a week. Visual testing revealed 20/200 acuity in OD with pinhole correction, and 20/30 in OS. Tonometry showed a pressure of 18 mm Hg in OD and 15 mm Hg in OS, and slit lamp examination was largely unremarkable. CRP was within normal limits, ESR was unknown, and D-dimer was elevated at 1.64 mg/L. COVID-19 test was negative. MRI of the brain and orbits with and without contrast showed diffuse bilateral periventricular and deep white matter hyperintense foci on T2 and T2 FLAIR sequences, possibly related to microvascular disease. No evidence of diffusion restriction, hemorrhage, edema, or intracranial mass was identified and bilateral orbits and optic nerves appeared normal. CT of the head without contrast showed right-sided anterolateral soft tissue scalp swelling and hemorrhage due to recent trauma as well as bilateral athero-sclerotic calcification of the carotid arteries. Notably, CT scan of the chest with contrast showed a right lower lobe pulmonary nodule measuring up to 9 mm, suspicious for possible malignancy. Right-sided temporal artery biopsy was negative for pathological findings indicative of giant cell arteritis. The patient was started on prednisone PO 50 mg daily as well as tocilizumab for presumptive biopsy-negative giant cell arteritis. Exam remained unchanged upon one month follow-up. Differential diagnoses included biopsy-negative giant cell arteritis, traumatic optic neuropathy, and infiltrative optic neurop-athy secondary to infectious etiology or malignancy given the context of the patient’s newly detected pulmonary nodule and positive PPD status.
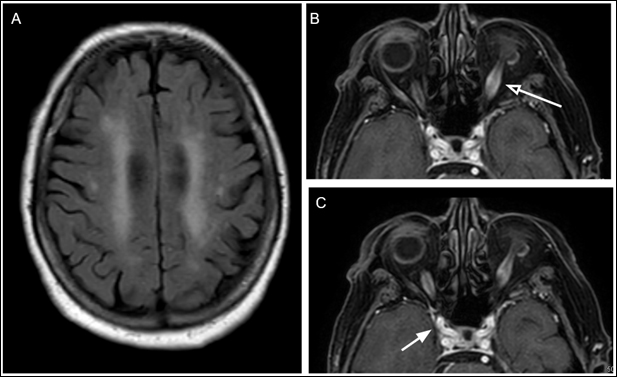 Figure 6: MRI of the brain and orbits with and without contrast of patient discussed in Case 8. A.) Axial T2 FLAIR image showing nonspecific likely age-related areas of hyperintense foci. B.) and C.) Axial T1 weighted post-contrast fat-saturated image showing bilateral optic nerve sheath dilation and enhancement, more prominent on the left side (white arrow with clear arrowhead). Optic nerve sheath dilation extends to intracranial and chiasmal portions of the optic nerve bilaterally (white arrow with solid arrowhead). Inflammatory soft tissue changes also noted in the right preseptal compartment, likely secondary to recent head trauma.
Figure 6: MRI of the brain and orbits with and without contrast of patient discussed in Case 8. A.) Axial T2 FLAIR image showing nonspecific likely age-related areas of hyperintense foci. B.) and C.) Axial T1 weighted post-contrast fat-saturated image showing bilateral optic nerve sheath dilation and enhancement, more prominent on the left side (white arrow with clear arrowhead). Optic nerve sheath dilation extends to intracranial and chiasmal portions of the optic nerve bilaterally (white arrow with solid arrowhead). Inflammatory soft tissue changes also noted in the right preseptal compartment, likely secondary to recent head trauma.
The ocular and neurological complications of COVID-19, as well as its various routes of viral entry, are not yet completely elucidated. SARS-CoV-2 has the potential to invade the CNS, as the virus has been detected in the CSF of confirmed COVID-19 patients [3]. Viruses from the Coronaviridae family, which includes SARS-CoV-2, have also been shown to be capable of causing anterior uveitis, choroiditis, vasculitis, and retinitis in feline and murine models, although this has not yet been definitively demonstrated in humans [13]. It has been reported that acute and subacute neurological complications of SARS-CoV-2 infections have been found in up to 85% of patients, both in those with severe disease and minimally symptomatic or asymptomatic patients [4]. SARS-CoV-2 has been hypothesized to enter the central nervous system via hematogenous and/or retrograde neuronal spread [5]. Multiple mechanisms of entry have been proposed, including direct penetration of the blood–brain barrier (BBB) as well as infection of the endothelial microvascular cells that express the angiotensin-converting enzyme 2 (ACE-2) [5]. ACE-2 receptors are also known to be expressed in the aqueous humor as well as the retina, which supports the postulation that SARS-CoV-2 can directly involve the eye [3], as the virus has been hypothesized to spread through conjunctival mucous membranes. Neuronal retrograde spread has also been conjectured to occur through olfactory or enteric nerves [5]. Finally, the resulting cytokine storm that is triggered by multi-system inflammation with SARS-CoV-2 infection can also result in direct compro¬mise to the BBB [6], and lead to the downstream neurologic, ocular, and olfactory manifestations that have been documented in COVID-19.
A variety of neuro-ophthalmological as well as ocular non-neurological manifestations of SARS-CoV-2 infection have been repeatedly documented in the existing literature. Some report¬ed neuro-ophthalmological presentations include optic neuri-tis, cranial nerve palsies, vision loss, nystagmus, eye movement abnormalities, and visual field defects, while ocular symptoms that have been commonly described include conjunctivitis, keratoconjunctivitis, uveitis, and retinitis [10,11]. One case series involving 38 patients published by Wu et al reported a 31.6% prevalence of ocular findings such as chemosis, epiphora, conjunctival hyperemia, and secretion in patients with SARS-CoV-2 infection in Hubei province, China [12]. The findings of the study also suggested that the presence of ocular symptoms appeared to be correlated with more severe laboratory abnormalities such as higher white blood cell and neutrophil counts, and more elevated C-reactive protein lactate dehydrogenase, and procalcitonin levels [12]. Another study involving retinal examinations of twelve COVID-19 patients performed by Marinho et al found that all twelve patients exhibited bilateral hyperreflective lesions in the ganglion cell and inner plexiform layers on optical coherence tomography (OCT) [14]. Four patients in the study were also found to have cotton wool spots and retinal hemorrhages on fundoscopic exam [14]. It is still unclear whether SARS-CoV viruses have the ability to be transmitted through ocular secretions and membranes. A 2004 study showed that SARS-CoV RNA was identified in the tears of three out of 36 SARS patients via RT-PCR amplification [15], suggesting a possible mechanism of viral entry through an ocular route. Whether this is through direct viral inoculation, migration of the virus through the upper respiratory tract to the nasolacrimal duct, hematogenous spread to the lacrimal ducts, or some other route yet to be described remains unclear [15]. However, these findings combined with multiple reports of ocular findings in COVID-19 patients suggest poten-tial spread of the virus through ocular membranes and tissues, and emphasize the need for a more detailed understanding of SARS-CoV-2 routes of infection to guide infection control and public health measures.
Cranial nerve palsies, ophthalmoplegia, nystagmus, visual field defects, and optic neuritis are some of the more commonly reported neuro-ophthalmological manifestations in confirmed COVID-19 patients. These have been described to occur independently, as well as in conjunction with acute im¬mune-mediated polyneuropathies such as Guillain-Barré syndrome and Miller-Fisher syndrome [10,11]. There have also been reports of myelin oligodendrocyte glycoprotein (MOG) antibody positivity in patients with confirmed or presumed SARS-CoV-2 infection presenting with visual loss and ocular pain [16]. MOG antibodies are typically seen in CNS inflammatory disorders such as multiple sclerosis and neuromyelitis optica, which commonly present with optic neuritis. Additionally, cranial nerve deficits such as ptosis and diplopia have also been commonly reported [10]. One report of a pediatric case of COVID-19 complicated by multisystem inflammatory syndrome in children (MIS-C) also described symptoms of papilledema, headache, abducens palsy, and elevated CSF opening pressure consistent with secondary pseudotumor cerebri syndrome [17]. Oscillopsia, ataxia, myoclonus, and other encephalitis-like symptoms have also been reported in patients following SARS-CoV-2 infection. These findings were presumed to be secondary to post-infectious immune-mediated encephalitis, which were consistent with cerebellar lesions seen on MRI [10]. Third and sixth nerve palsies have also been described following resolution of COVID-19 infections, consistent with Miller-Fisher syndrome, and other cases of peripheral neuropathies without eye involvement consistent with Guillain-Barré syndrome have also been reported [11]. As with current reports of multisystem inflammatory syndrome thought to be linked to COVID-19, further studies are needed to clarify whether these neurological and systemic syndromes described following acute SARS-CoV-2 infection are the direct result of SARS-CoV-2 virulence, or more consistent with overall systemic immune dysregulation.
The incidence of large vessel vasculitides such as giant cell arteritis (GCA) has also been noted to be significantly more prevalent during the COVID-19 pandemic [7]. While an increase in preventable bilateral blindness secondary to GCA has been attributed to delays in diagnosis during the pandemic in some studies [8], other reports show a substantial increase in the incidence of GCA during the COVID-19 pandemic without any significant delays in GCA diagnosis and treatment. One institution in France reported a 70% increase in incidence of GCA in 2020 during the COVID-19 pandemic compared to the previous year, which closely reflects our experience this year in our practice [7].
It has been established that the etiology of GCA involves damage to the adventitial layer of three-layered vessels due to entry of macrophages and T-cells through the vaso vasorum after an initial inflammatory trigger, resulting in subsequent destruction and remodeling of the vessel wall. These inflammatory events subsequently result in the ischemic symptoms such as vision loss, jaw claudication, and headache that are the hallmarks of GCA. The trigger for the inciting inflammatory event is unknown; a wide range of genetic, infectious, and environmental factors have all been proposed. It is plausible that the underlying systemic inflammation caused by SARS-CoV-2 infection can play a role in the increase in GCA incidence re-ported at some institutions.
The neuro-ophthalmological outcomes of SARS-CoV-2 infection are not well understood, both in the context of preexisting GCA and Takayasu Arteritis (TA) and without preexisting large-vessel vasculitis. One monocentric survey at an institution in Italy studied the impact of superimposed SARS-CoV-2 infection in patients with GCA and TA. Their results indicated no changes in large-vessel vasculitis relapse rates or adverse COVID-19 outcomes [8]. Out of 67 patients with TA and 95 patients with GCA interviewed in the study, none had clinical large-vessel vasculitis relapses [8]. Of the two GCA and two TA patients diagnosed with SARS-CoV-2 infection, all made a full recovery and none required oxygen support [8]. Due to the limited sample sizes however, no definitive conclusions can be drawn. Another study described two cases of severe bilateral vision loss in two patients with COVID-19 without preexisting GCA [9]. Both patients experienced thrombotic events confirmed on neuroimaging, which was determined to be the cause of their visual loss. As evidenced by greatly elevated D-dimer levels, ESR, and CRP in one of the patients, the systemic inflammation caused by COVID-19 could result in subsequent activation of coagulation, leading to a hypercoagulable state and ischemic complications. How this proposed increased susceptibility to thromboembolic events relates to large-vessel vasculitis outcomes have yet to be fully elucidated, and how different environmental and genetic factors affect these outcomes have yet to be defined.
The diverse manifestations of COVID-19 continue to pose challenges in clinical diagnoses and management. We present seven cases of patients exhibiting unusual neuro-ophthalmological symptoms — three patients testing negative for COVID-19, and four with unknown COVID-19 status — in which the possibility of SARS-CoV-2 infection complicated the clinical picture. Given the unknown true prevalence of the novel Coronavirus Disease-2019 despite increased testing efforts, as well as reports of high proportions of asymptomatic carriers, it is plausible that the rise in unusual neuro-ophthalmological cases observed during the pandemic are associated with undiagnosed COVID-19. Further investigation into the mechanisms of viral entry into the CNS, as well as the pathophysiology underlying neurological manifestations of COVID-19 is needed to gain a better understanding of the virulent and infective properties of SARS-CoV-2 and provide insight into its most common neuro-ophthalmological presentations and potential ocular routes of viral transmission.
Acknowledgement: The authors have no conflicts of interest to declare and there is no financial interest to report.
- Douglas KAA, Douglas VP, Moschos MM (2020). Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature. In Vivo, 34(3 suppl):1619-1628. doi:10.21873/invivo.11952
- Wood H (2020). New insights into the neurological effects of COVID-19. Nature Reviews Neurology, 16(8):403-403. doi:10.1038/s41582-020-0386-7
- Baig AM, Sanders EC (2020). Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19). Journal of Medical Virology, 92(10):1845- 1857. doi:10.1002/jmv.26105
- Merello M, Bhatia KP, Obeso JA (2020). SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. The Lancet Neurology, 11. Doi: 10.1016/S1474-4422(20)30442-7
- Raony Í, de Figueiredo CS, Pandolfo P, Giestal-de-Araujo E, Oliveira-Silva Bomfim P, Savino W (2020). Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Frontiers in Immunology, 11. doi:10.3389/fimmu.2020.01170
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet, 395(10229):1033-1034. doi:10.1016/s0140- 6736(20)30628-0
- Lecler A, Villeneuve D, Vignal C, Sené T (2020). Increased rather than decreased incidence of giant-cell arteritis during the COVID-19 pandemic. Annals of the Rheumatic Diseases, annrheumdis-2020-218343. doi:10.1136/ annrheumdis-2020-218343
- Monti S, Delvino P, Bellis E, Milanesi A, Brandolino F, Montecucco C (2020). Impact of delayed diagnoses at the time of COVID-19: increased rate of preventable bilateral blindness in giant cell arteritis. Annals of the Rheumatic Diseases, 79(12):1658-1659. doi:10.1136/annrheumdis-2020-217915
- Cyr DG, Vicidomini CM, Siu NY, Elmann SE (2020). Severe Bilateral Vision Loss in 2 Patients With Coronavirus Disease 2019. Journal of Neuro-Ophthalmology, 40(3):403-405. doi:10.1097/wno.0000000000001039
- Gold DM, Galetta SL (2020). Neuro-Ophthalmologic Complications of Coronavirus Disease 2019 (COVID-19). Neuroscience Letters, 135531. doi:10.1016/j. neulet.2020.135531
- Luís ME, Hipólito-Fernandes D, Mota C, et al (2020). A Review of Neuro-Ophthalmological Manifestations of Human Coronavirus Infection. Eye and Brain, 12:129- 137. doi:10.2147/eb.s268828
- Wu P, Duan F, Luo C, et al (2020). Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmology, 138(5):575. doi:10.1001/jamaophthalmol.2020.1291
- Seah I, Agrawal R (2020). Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocular Immunology and Inflammation, 28(3):391-395. doi:10.1080/09273948.2020.1738501
- Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R (2020). Retinal findings in patients with COVID-19. The Lancet, 395(10237):1610. doi:10.1016/ s0140-6736(20)31014-x
- Loon S-C (2004). The severe acute respiratory syndrome coronavirus in tears. British Journal of Ophthalmology, 88(7):861-863. doi:10.1136/bjo.2003.035931
- Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR (2020). Myelin Oligodendrocyte Glycoprotein Antibody–Associated Optic Neuritis and Myelitis in COVID-19. Journal of Neuro-Ophthalmology, 40(3):398- 402. doi:10.1097/wno.0000000000001049
- Verkuil LD, Liu GT, Brahma VL, Avery RA (2020). Pseudotumor cerebri syndrome associated with MIS-C: a case report. The Lancet, 396(10250):532. doi:10.1016/ s0140-6736(20)31725-6.













