Article / Opinion Article
National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria
Prof. Dr. Hristo Odisseev
National Centre of Infectious and Parasitic Diseases
Bulgaria
17 August 2020 ; 15 September 2020
It is generally accepted that meningeal reactions in patients with mumps are due to the direct involvement of the meninges by the mumps virus. With the development of mumps vaccines, this view was extended to vaccinated people, who are considered serious post-vaccine meningitis. In present article, the author states that these reactions are not due to inflammation of the meninges, but to the choroid plexus caused by virulent and vaccine strains. Inflammation leads to an increase in cerebrospinal fluid secretion, which increases intracranial pressure and is manifested by meningeal symptoms. In the presence of this evidence, the author considers that meningeal reactions are not meningitis, but meningisms, based on clinical data, experiments on monkeys and the glymphatic system.
Key words: mumps virus, choroid plexus, meningitis, glymphatic system, meningism
It is widely known that the mumps virus has tropism to the excretory cells of mumps, testes, ovaries, pancreas and choroid plexus. Mumps virus has on its surface antigens that correspond to receptors located on the surface of the excretory cells of said organs. The virus does not affect cells with endocrine function that produce the enzymes insulin, amylase, testosterone, of the above mentioned organs, as they do not have similar receptors. The meningitis is considered a widespread and serious reaction to mumps infection. This type of meningitis is explained by the direct attack of the virus on the meninges. After the creation of mumps vaccines strains, this opinion is addressed to them. In the past, mumps infection was pandemic. Despite the successes obtained through vaccine prophylaxis, some aspects of the characteristic of the infection remains unknown. One of them is the pathogenesis of mumps meningitis. This gives to author an opportunity to discuss it in this article.
But before the discussion, it needed to get acquainted first the elements that make part in the pathogenesis. Structure and functions of the Meninges, Ventricles, Choroid plexus, and Cerebrospinal fluid.
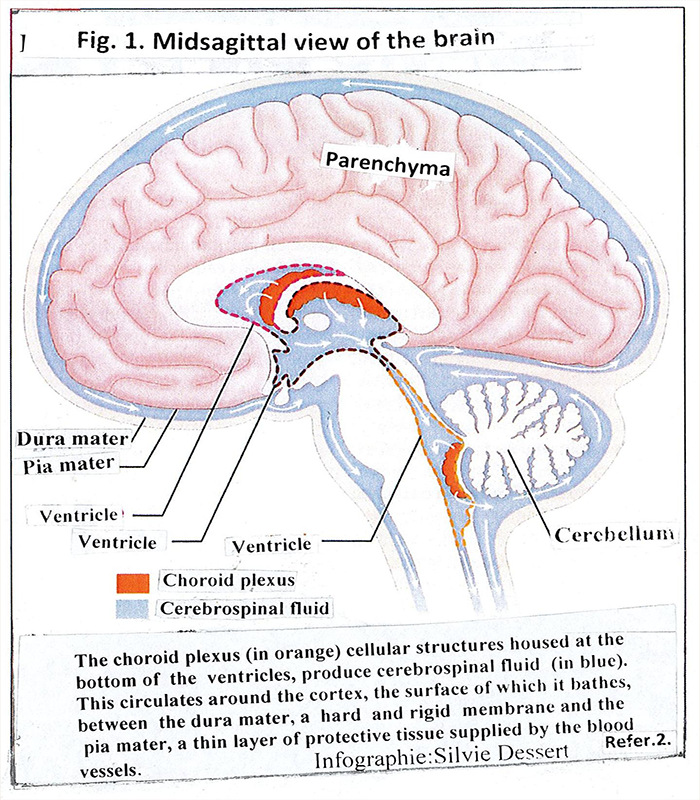
Figure 1: Shows the components meninges, ventricles, choroid plexus and cerebrospinal fluid
Meninges are composed of three layers: dura mater, arachnoid and pia mater, located on top of each other [1]. They form the external barrier of the brain, which mechanically protects it from the external space. There is a narrow space between the dura mater and the arachnoid that is filled with water, fibers and nerves. Between the arachnoid and the pia matеr the cerebrospinal fluid and the cerebral blood vessels pass.
The choroid plexus is composed of small plaques of epithelial cells located on the basal wall of the four ventricles of the brain [2] [Fig.1].They occupy about 0.25% of brain tissue. The brain of adults weighs just over 1 kg, choroid plexus 2-3 grams. Its main role is to produce cerebrospinal fluid. One gram of tissue produces about 4 ml of cerebrospinal fluid per minute. An amount is collected in 4 hours, which allows the cerebrospinal fluid to change 3-4 times a day and night [3]. The structure and function of the choroid plexus as well as the role of the cerebrospinal fluid have been studied in great detail [3-7]. Therefore, we will focus on them briefly
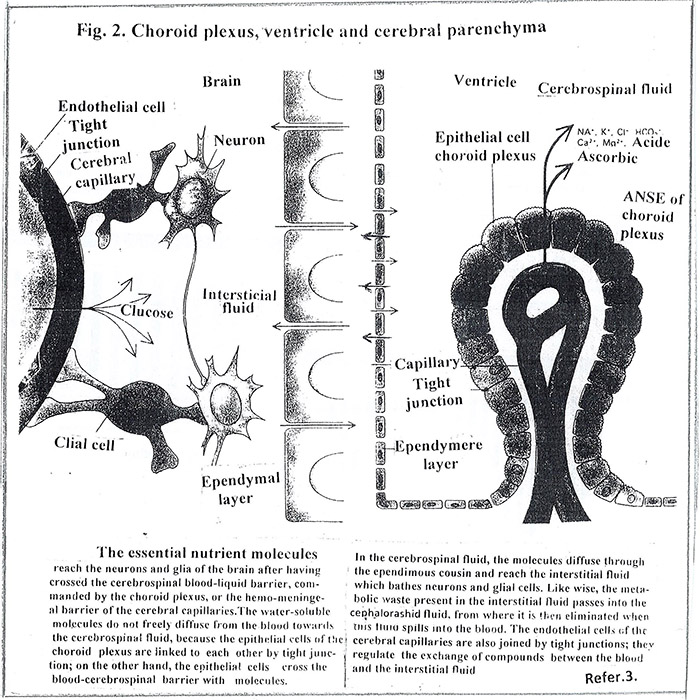
Under an optical microscope, the shape of the choroid plexus appears on a network of blood capillaries covert with cells [Fig.2]. The basal side of each cell is in close contact with the capillary wall and builds a blood-brain barrier. Between adjacent cells of the basal wall there are structures, (tight junction), which tighten the wall. This fact creates an internal barrier between the blood and the cerebrospinal fluid. The barrier functions actively: each cell drains from the blood ions, nutrients and other elements necessary for the normal functioning of the brain. It should be noted that the choroid plexus strictly and selectively commands the passage of molecules from the blood to the cerebrospinal fluid and ensures its chemical stability. The upper side of each cell is covered with microvilli which are rinsed in the cerebrospinal fluid, filling the ventricles. The internal barrier together with the external one creates complete isolation of the brain and contributes to stable homeostasis for its biochemical functions.
Unlike the epithelial cells of the choroid plexus, those that cover the rest of the ventricular walls, ependymal cells, are not connected to the tight junction, which allows intimate contact between the cerebrospinal fluid and the interstitial fluid of the brain parenchyma.
The cerebrospinal fluid performs many functions, two of which are the most important. The first: supplies the necessary bioenergy and nutritional materials to the brain parenchyma, (neural and glial cells), ensuring its normal functioning. The second: eliminates waste, resultant cell metabolism. The two functions acts simultaneously are carried out during the circulation of the cerebrospinal fluid around the brain.
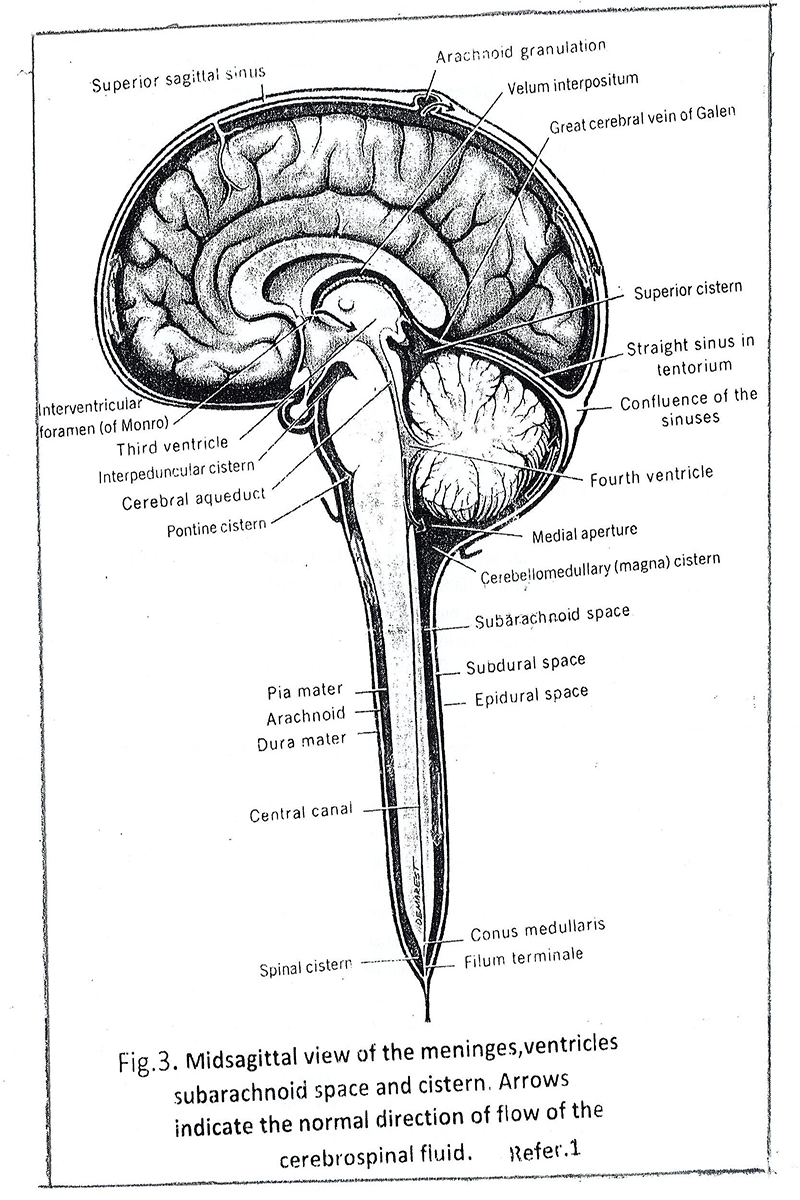
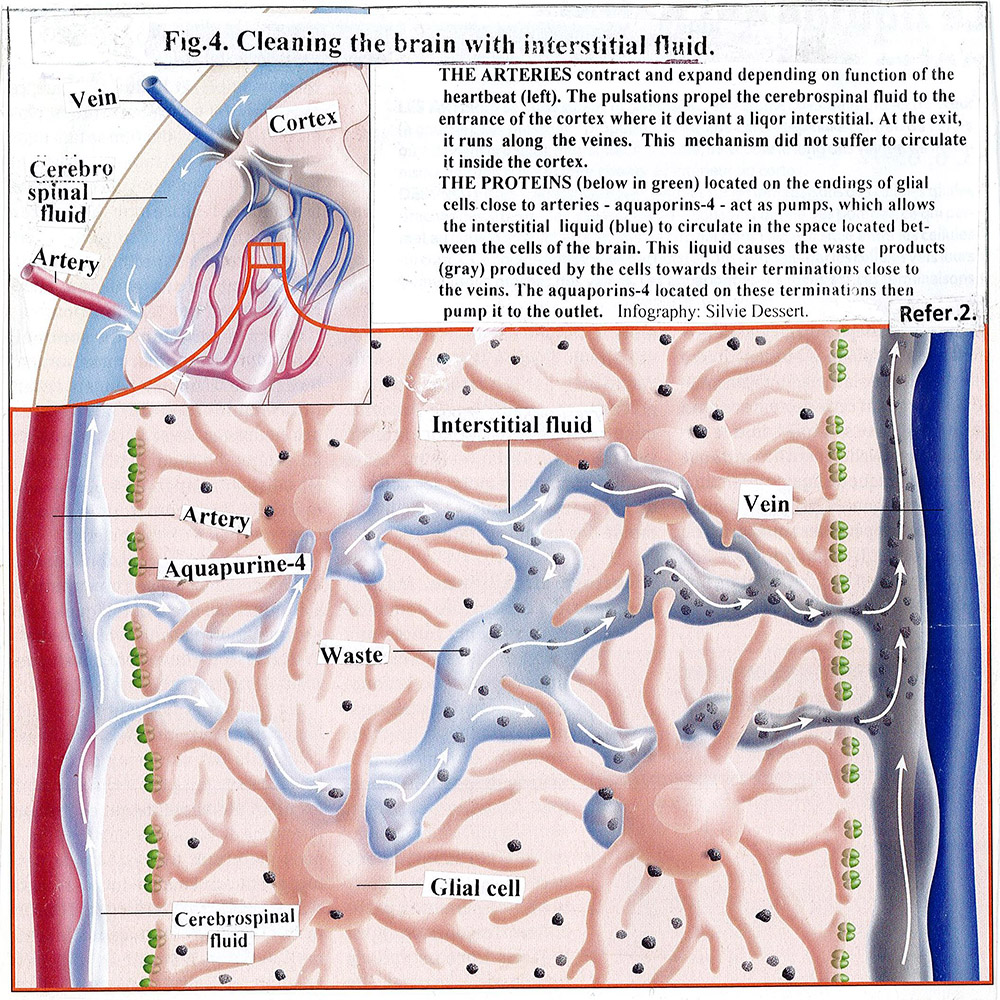
Cerebrospinal fluid leakage is a slow process. [Fig.3]. It is squeezed from fourth ventricle into a cistern magna of the subarachnoid space, moves slowly around the brain (including the spinal cord) and finally reaches the superior sagittal sinus and by arachnoid granulation leaves the cranial cavity. We mentioned above that the cerebral arteries and the cerebrospinal fluid pass through the subarachnoid space. The arteries make small twigs that penetrate deep into the brain and branches into capillaries. Interestingly, they pulsate (contract and expand) in sync with the heartbeat. Part of the cerebrospinal fluid penetrates in around arterial space and flows to the around capillary space and eventually passes into the parenchyma of the brain and becomes fresh interstitial cerebral fluid. The process is supported by two factors [2] [Fig.4]. The first: the aquapurines -4, located at the end of the glial cells that are close to the cerebral arteries, pumps cerebrospinal fluid and directs it to the around arterial space. The second: the permanent pulsations of the arteries and capillaries move the cerebrospinal fluid along the around arterial and capillary spaces to the inside of the brain. The fresh interstitial cerebral fluid pushes away old fluid that has already consumed the necessary energy and nutrient elements. It floods neural and glial cells and supplies them with new elements. The old fluid enters the around vein spaces and goes to the vein system. With its movement, it attracts from the intracellular space the waste secreted by the cells as a result of their metabolism and dragging them through the around small vein spaces to the nearby large veins. The aquapurines-4, located on the glial cell ending closed the veins, pushing the old fluid in them. This movement of the cerebrospinal fluid, entry and exit of the brain, called Glymphatic System by Danish neuroscientist Maiken Nedergraad, replaces the lack of the lymphatic system of the brain. Its perform is the function of cleansing the parenchyma of discarded waste products, resulting from metabolism, including amyloid betta (Aβ), which is considered as the cause of Alzheimer’s disease [8] [Fig.4].
Liff J.J. and team using in vivo two-photon microscopy and ex vivo a small fluorescent substances observe cerebral blood pulsations and the movement of cerebrospinal fluid around the arteries and prove how fresh cerebrospinal fluid enters, moves in the around arterial and capillary spaces and prove how the old interstitial fluid flows in the around vein spaces [9].
All these processes of the cerebrospinal fluid; entry, movement and exit of the brain parenchyma, prove that it makes direct contact with neural and glial cells.
Like any organ, the choroid plexus can be affected by various diseases: tumors, infected with bacterial, viral (including mumps virus) and other pathogens. When the pathogenic agent causes an inflammatory process, cerebrospinal fluid secretion increases.
The wild mumps virus, which enters in the body through the respiratory tract, travel to the regional lymph nodes and then to the bloodstream. It multiplies in the cells of the immune system (in lymphocytes) and causes viremia [10, 11].
This fact is proved by isolation of mumps virus from blood, saliva, urine and cerebrospinal fluid. The presence of viruses in the cerebrospinal fluid indicates that they overcome the internal barrier, pass through the capillary wall and make contact with the attached epithelial cells of the choroid plexus. Viral antigens bind to cell receptors and virus enter in the cell, multiply and cause inflammation. This process increases the amount of cerebrospinal fluid secretion and confuses its dynamics. The increased amount of cerebrospinal fluid swells the ventricles. The offspring of the virus pass into the cerebrospinal fluid.
As mentioned above, in the circulation of cerebrospinal fluid around the brain, part of it passes through the glymphatic system into the parenchymal space of the brain becomes interstitial fluid, but in increased amount.
The increased amount of cerebrospinal fluid in the ventricles and the brain parenchyma increases intracranial pressure and causes meningism, expressed by the triad of symptoms: stiff neck, headache and temperature.
During the stay in the brain parenchyma, mumps viruses come into contact with neural and glial cells. However, they do not enter in them because the latter do not have the appropriate receptors to correspond to the viral antigens. They are not excreted cells.
Once the inflammation of the choroid plexus is complete, its secretion returns to normal, the intracranial pressure decreases and the meningeal symptoms disappears without any consequences.
The strains of mumps vaccines are alive but attenuated and after vaccination entering the body they repeat the path of the wild mumps virus. However, the course of the inflammatory process caused by them in the choroid plexus is significantly lower, the intracranial pressure is much lower and the neurological symptoms are many time rarer, mild, transient, and their manifestation is incidental.
The degree of inflammation of the choroid plexus depends on the degree of attenuation of the strain, the amount of virus in the blood (dose of the vaccine), the age of the vaccinated and the intensity of the immune system.
In the past, mumps was one of the mandatory child’ infectious. It had been picking on children from an early age and had almost exhausted her contingent by to adolescence. It is a relatively mild disease, but with complication “aseptic meningitis”, and single orchites and oophorites of person in the sexually mature, who have missed the opportunity to become ill as young. According to the literature data, the number of patients with mumps meningitis ranged from 20% to 30%. But there were also authors’ opinions up to 100%, refer to the cerebrospinal indicators: high-pressure cerebrospinal fluid leakage with lumbar puncture and regular pleocytosis.
The mild symptoms, rapid resolution and without any consequences did not attract the attention of researchers to the pathogenesis of the mumps meningitis and don’t studies it in detail. Their authority rose suddenly after the creation of mumps vaccines, mono, then in association with measles, mumps and rubella (MMR) in which also began to appear “meningitis”, although much rare and milder than natural infection.
The mumps meningitis were become an important indicator of the virulence of vaccine strains and the reactivity of vaccines. They have become an obstacle to the introduction of vaccines into practice for several decades. Even vaccines already approved, included in national vaccine programs, regularly used in practice and with high epidemiological and economic effect have rejected [12].
Intensive studies on the virulence of strain and vaccines have been launched worldwide. Unfortunately, only in one direction: the vaccine strains [13, 14]. The sequence of the genome of the vaccine strains and the comparison with wild ones, to determine the nucleic segments responsible for their virulence did not give an effect [15-18]. The initiator of the Bulgarian vaccines, mono and in the combination measles-mumps (Former specialist in infectious diseases, who passed in the field of virology due to hearing loss), head of the Research-Production Laboratory of measlesmumps-rubella at the Research Institute of Epidemiology and Microbiology (RIEM), Sofia, Bulgaria, have no conditions for molecular studies. Based on his knowledge of the clinic of mumps infection, he set out on another path: clarified the pathogenesis of mumps meningeal reactions. He is familiar with the tropism of the virus to the excretory cells of the parotid, pancreatic, testicular and choroid plexus and the clinic of mumps.
Now he must make his knowledge the structure of the meninges and plexus choroid and its function for the production of cerebrospinal fluid and its circulation. And after getting acquainted with them, he formulated the following conclusions:
- The role of the choroid plexus is in accordance with the clinical observation of vaccinated individuals showing meningeal symptoms in contradiction with clinical symptoms and liquor findings: mild clinical sign, mainly neck stiffness and headache against clear liquor, outflow under pressure with puncture, with higher pleocytosis and increase proteins.
- In meninges there is no condition for reproduction of the mumps virus.
- Neuronal and glial cells are not secretory and not have receptors corresponding to viral antigens.
This gives the author reason to formulate the opinion:
In natural infection and vaccination, meningitis is not due to direct involvement of the meninges, but to the choroid plexus from the mumps virus. Inflammation of the choroid plexus leads to increase secretion of the cerebrospinal fluid. Abundant cerebrospinal fluid increases intracranial pressure, which is clinically manifested by meningisms. These neural symptoms, with a mild course and without any consequences, are inevitable but acceptable.
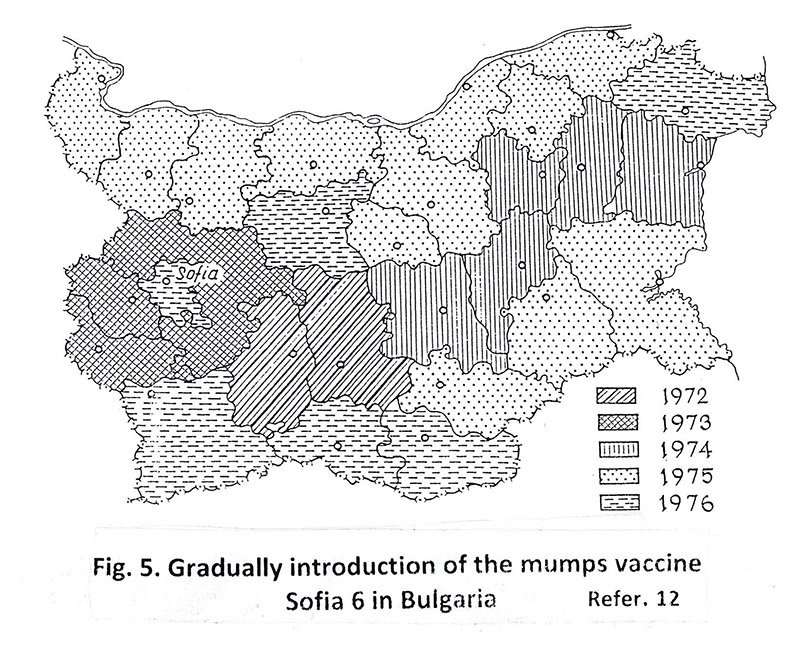
This opinion, which the author defends firmly, gives him possibility to create a Bulgarian mumps vaccine, Sofia-6 (1969) and the first in the world to start applying it in practice as early as 1972. [Fig.5].
Later, the absence of receptors on the surface of the neural and glial cells for corresponding to mumps antigens was confirmed in experiments conducted in collaboration with the Institute for Production Viral Vaccines, Moscow, and the Institute of Virology in East Berlin. More 100 monkeys were inoculated with the mumps vaccines Leningrad-3, Sofia-6, Jeryl Lynn [13, 14]. It should be added that in the experiment, the vaccines were administered directly to the monkeys’ brain, but none of the animals developed encephalitis, meningitis, or other neuronal symptoms that were clinic or pathologic – anatomic confirmed.
Ten year ago, the glymphatic system categorically confirmed the absence of receptors in neuronal and glial cells against mumps antigens [8]. If they have receptors corresponding to mumps virus antigens, during the stay of infected with the virus cerebral fluid in the brain parenchyma of patient or vaccinated, the neurological symptoms would have a completely different form.
Throughout the decades-long debate on the virulence of vaccine strains and the reactivity of vaccines, the WHO has taken a neutral position. She left it to local health authorities themselves decides how to use their vaccines. This tactic of WHO gave the author possibility to create the Bulgarian mumps vaccine Sofia-6 and to start its application in practice as early as.
Only, in February 2007, the GACSV in WHO published its opinion: in terms of safety, all mumps vaccine prepared for which relevant data are available and acceptable for use in an immunization program [19].
The problem of mumps vaccines was cancelled and it does not present in the future plans of the WHO. From now on they included in the combined measles-mumps-rubella (MMR) and measles – mumps – rubella- varicella (MMRV) vaccines and distributed in parallel with them However, the proposal to replace the term meningitis with meningism was not considered.
For example, in Bulgaria, the leaflet for routine use of the MMR vaccine in the section “Possible side effects” in the first place is “aseptic meningeal syndrome” [20]. Some parents consider it a serious syndrome and do not vaccinate their children.
The problem is also scientifically important. The term mumps meningitis continues to be used in the scientific, educational and public literature, which does not correspond to the objective facts. It is time for the WHO or relevant Institution to provoke a discussion and make a formal decision to replace the term “mumps meningitis” with term “mumps meningism” [21].
This will put end of the scientific inaccuracy that has survived for centuries.
- Noback CB, Strominger NL, Demarsted RJ (1996). Meninges, Ventricles and Cerebrospinal Fluid. In Human Nervous System – Structure and Function, 5 edition. Williams & Wilkins. NY, 68-74.
- Bernard Ch (2014). Le liquide qui nettoie notre cerveau. La Recherche, September, no 491: 56-60.
- Spector R, Johanson C (1992). “Les reins” du cerveau. Pour la science, No 147: 82-89.
- Johanson CE, Stopa EG, McMillan PN (2011). The blood-cerebrospinal fluid barrier structure and functional significance. Methods Mol Biol, 686: 101-31.
- Johanson CE, Duncan JA, Klinge PM, Brinker T. Stopa EG, Silverberg, GD (2008). Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Research, 5: 10.
- Spector R, Keep RF, Robert Snodgrass S, Smith QR, Johanson CE (2015). A balanced view of choroid plexus structure and function: Exp Neurol, 267: 78-86.
- Spector R, Robert Snodgrass S, Johanson CE (2015). A balanced view of the cerebrospinal fluid composition and functions. Exp Neurol, 273: 57-68.
- LIiff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H et al (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid Аβ. Sci Transl Med, 4(147): 147ra111.
- LIiff JJ, Wang M, Zeppenfeld DM, Vencataraman A, Plog BA, LiaoY, Dean R, Nedergaard M (2013). Cerebral arterial pulsation drives paravascular CSF – interstitial fluid change in the murine brain. J Neurosci, 33(46):
18190 -18199. - Overman JR (1958). Viremy in human mumps infection. Arch Inter Med, 102: 345-356.
- Fleischer B, Kroeth HV (1971). Lymphoid mumps virus replication in human lymphocytes cell lines and in peripheral blood: preference T cells. Infect Immun, 35: 25-31.
- Odisseev H, N. Gacheva (1994). Vaccinoprophylaxis of mumps using mumps vaccine, strain Sofia 6 in Bulgaria.
Vaccine, 12(14): 1251-1254. - Rosina EE, Captzova TI, Andzaparidze SG, Odisseev H, Jukovez V, Todorova H (1978). Comparative morphologic study of the neurovirulence of vaccinal mumps strаins Leningrad-3 and Sofia 6. Epidemiol Microbiol Infect Dis, 15: 174-179.
- Rosina EE, Hilgenfeldt M (1985). Comparative study on the neurovirulence of different vaccine strains of mumps in monkeys. Acta Virologica, 29: 225-230.
- Forsey T, Mawn JA, Yates PhJ, Bentley ML, Minor PD (1990). Differentiation of vaccine and wild mumps viruses using the polymerase chain reaction and dideoxynucleotide sequencing. J Gen Virol, 71: 987-990.
- Yamada A, Takeuchi K, Tanabayashi K, Hishivama M. Takahashi Y, Sugiura A (1990). Differentiation of the mumps vaccine strains from the wild viruses by the nucleotide sequences of the P gene, 8: 553-556.
- Brown EG, Furesz J, Dimock K, Yarosh W, Contreras, G (1991). Nucleotide sequence analysis of Urabe mumps vaccine strain that caused meningitis in vaccine recipients. Vaccine, 9: 840-842.
- Katayama k, Oya A, Tanabayashi K, Okazaki K, Hishiyama M, Yamazaki S, Yamada A (1933). Differentiation of mumps vaccine strains from wild viruses by single-strand conformation polymorphism of the P gene. Vaccine, 11: 621-623.
- WHO, GACVS (2007). Position paper. Mumps virus vaccines. WER, 82(7): 58-60.
- M-M-RVAXPRO. Measles, mumps, and rubella vaccine (live). Merck Charp and Doum Bulgaria. EOOD. Info –msdbg@merck.com.
- Wikipedia. Meningism. en.wikipedia.org./wiki/meningism.