Article / Opinion Article
Univ. Manouba, ISBST, BVBGR-LR11ES31, Biotechpole of Sidi Thabet, Ariana, Tunisia.
Mohamed Neifar
Enzymologist
Univ. Manouba
Biotechpole of Sidi Thabet
Ariana
Tunisia
14 August 2020 ; 7 September 2020
The global Covid-19 pandemic and cross-contamination in hospitals has led to a serious public health problem and severe economic consequences [1]. As the Coronavirus panic continues to spread across the world, engineers and scientists are working hard to find new ways of developing novel respiratory protective devices. In the context of this prevention, the creation of antimicrobial textiles for medical applications (masks, gloves, surgical gowns …) appears an urgent necessity to fight against infections caused by pathogenic viruses and bacteria. Antimicrobial agents are molecules with the ability to kill microorganisms (biocides) or prevent their growth (biostatics). There are many antimicrobial molecules that can be used for the functionalization of textiles, the most widely used in the field of textiles are triclosan and its derivatives, zeolites (silver and copper or silver and zinc aluminosilicates), quaternary ammoniums, mineral powders (silver and copper), phenols, polyphenols, chitosan, silver ions, antimicrobial peptides and lytic enzymes [2-5].
Antimicrobial peptides and enzymes are proteins whose basic structural units are amino-acids. These amino-acids are joined by peptide bonds to form polypeptide chains. Some radicals of the amino acids have NH2 functions allowing their immobilization by covalent bonds on insoluble supports such as cotton cellulose [6-8]. For covalent bond formation, a prior activation of the cellulosic fibre networks is necessary. Numerous physico-chemical processes exist and are exploited to improve the properties of cellulosic fibrous materials. The chemical approach involves selective oxidants towards the cellulose hydroxyl functions such as sodium periodate (NaIO4), nitrogen peroxide (N2O4), hydrogen peroxide (H2O2), sodium hypochlorite (NaClO), ozone (O3); and 2,2,6,6-Tetramethylpiperidine-1-oxyl.radical (TEMPO). Among these, sodium periodate oxidation is characterized by its simplicity of use and its commercial success. Indeed, sodium periodate remains one of the most selective oxidants in sugar chemistry. It allows a C2-C3 cycle opening with functionalization at the same sites (Figure 1). Another (physical) approach can be envisaged with the use of cold plasmas. Indeed, some of these partially excited gases react with cellulose and can give rise to oxidation reactions [6-8].
Several antimicrobial enzymes and peptides have been immobilized on cellulose for application in the medical, sports and food industries. The immobilization on synthetic supports generally brings a possibility of cyclic use, a better mechanical and thermal stability as well as an easier control of the kinetic parameters [9-11].
Proteases and nucleases have been used to degrade proteins and nucleic acids of viral particles. Ribonucleases (or RNAases) catalyze the degradation of RNA. They are very stable and active and generally do not require co-factors to function. RNAases are difficult to inactivate, and usually minute amounts are sufficient to destroy viral RNA. Trypsin, chymotrypsin and proteinase K are digestive proteases that are effective in breaking down viral capsids. Trypsin cleaves proteins after the positively charged amino acids lysine or arginine, chymotrypsin after aromatic or hydrophobic amino acids such as tyrosine, phenylalanine or leucine. Proteinase K is an enzyme of the serine protease family. It is commonly used in molecular biology to digest proteins and remove contaminants from nucleic acid preparation. The use of proteases and immobilized nucleases for biotechnological applications is widely studied in the literature and is the subject of several international patents [12-17].
Antimicrobial peptides generally contain between 12 and 50 amino acids. These peptides exert a variety of antimicrobial activities, ranging from membrane permeabilization to action on a range of cytoplasmic targets. They have shown bactericidal effects against the most threatening bacteria to human health such as “Acinetobacter”, “Pseudomonas”, and various enterobacteria (including “Salmonella”, “Klebsiella“,“E. coli”, “Serratia”, and “Proteus”). Nisin is a polycyclic antibacterial peptide with 34 amino acid residues. It is used as a food additive (preservative) under the number E234. Nisin is produced by the lactic acid bacterium Lactococcus lactis. It is a type A lantibiotic that dissipates proton-motor force through pore formation and interferes with peptidoglycan synthesis. While most bactericides generally only inhibit closely related species, nisin is a rare example of a broad spectrum bactericide that is effective against many Gram-positive bacteria. The antiviral activity of nisin has been well documented. Lactoferrin has a broad antimicrobial spectrum ranging from Gram- and Gram+ bacteria, yeasts, fungi, to certain viruses and protozoa. The main mechanism by which it exerts its bacteriostatic action is iron deprivation. Lactoferrin also exerts bactericidal activity independent of its iron chelating function. Through its ability to bind directly to LPS and lipoteichoic acids, lactoferrin destabilizes the membrane of bacteria, causes their fragility and increases their permeability. It exerts biocidal activity against DNA and RNA viruses, particularly those of hepatitis, herpes and HIV. The mechanism of lactoferrin’s antiviral action is not fully elucidated. Nevertheless, in most in vitro studies, lactoferrin inhibits virus attachment and entry to host cells. Lactoferrin also appears to have the potential to inhibit viral replication of HIV-1, chronic hepatitis C and rotaviruses. Early clinical trials showed that oral administration of lactoferrin increased the reduction in viral load in Herpes virus-infected patients treated with acyclovir [18-25].
In conclusion and based on this bibliographic search and analysis, it is theoretically and technically possible to design and manufacture a sophisticated virucide face mask, which will be able to degrade the virus components, particularly its genetic material (RNA) and its capsid (consisting essentially of proteins) (Figure 2). Degradation will be achieved by commercial enzymes (proteases and RNAases) immobilized on cellulose, a major component of cotton masks. The performance of this bioactive face mask will be easily evaluated by specific antiviral tests. In order to further improve its biocidal efficacy, antimicrobial peptides such as nisin (anti-Gram+) and lactoferrin (anti-Gram-) will also be co-immobilized. This mask will have the properties of being sufficiently virucidal, bactericidal, immunogene, totally harmless, reusable, comfortable and easy to wear in compliance with standard procedures. It will thus represent one of the best forms of protection for medical staff and target population against viral agents and pathogenic bacteria.
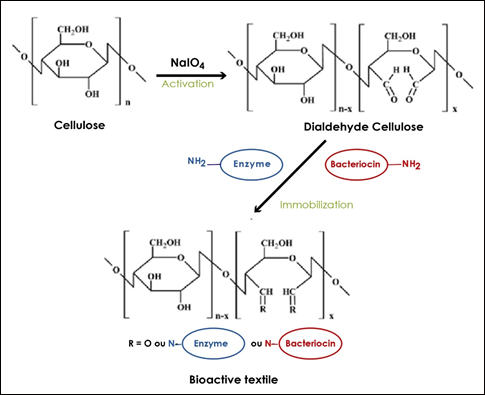
Figure1 : Activation and immobilization steps needed to obtain bioactive textile.
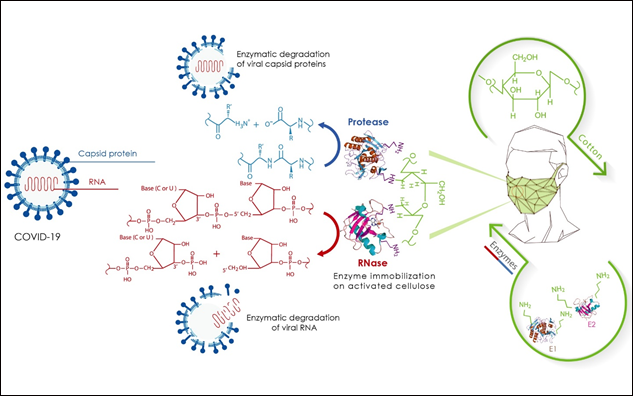
Figure 2 : Representative scheme illustrating the design of a virucidal face mask based on nucleasic and proteolytic enzymes immobilized after chemical activation of cotton cellulose with periodate.
This study was supported by the Tunisian Ministry of Higher Education and Scientific Research in the ambit of the laboratory project LR11ES31.
I would like to thank Mr Chaouki NEIFER, Medical Doctor and Cardiovascular Surgeon at Mercy Hospital (Metz, France)
with whom I have had a fruitful discussion which led to this work as well as another project that he will publish it soon.
Author declares that there are no conflicts of interest.
- Neifar M (2020) Necessity for designing multienzymestargeting drugs to fight Coronavirus. Adv Tissue Eng
Regen Med Open Access, 6(1): 6‒7. DOI: 10.15406/atroa.2020.06.00109 - Kazuo N, Yoshikazu T (1999) Gargling cup, antiviral mask, antiviral filter, antifungal, antibacterial, and antiviral filter air cleaner and air-cleaner humidifier, Patent US5888527, published 1999-03-30, Application US19970840633, 1997-04-25.
- Nashimoto K, Tashiro Y, Kosaka Y, Hara Y (1998) Antiviral filter air cleaner impregnated with tea extract/ use of tea extracts (tea polyphenol) having the effect of inactivating the viruses. Patent US5747053 published 1998-05-05, Application US1996-647012, 1996-05-09.
- Kirwan D (1977) Process for effecting enzymatic reactions in aerosol. Patent Number GB 1460975, 1977-01-06, Application number GB19740023976 19740530.
- Wen SH (2003) Antiviral and antibacterial respirator mask. Patent Number WO03/051460. Application number PCT/US02/40536, 2003-06-26.
- Liu Y, Chen J (2016) Enzyme immobilization on cellulose matrixes. Journal of Bioactive and Compatible Polymers, 31(6): 553-567
- Ibrahim N, Gouda M, El-shafei A, Abdel-Fatah O (2007) Antimicrobial activity of cotton fabrics containing
immobilized enzymes. Journal of Applied Polymer Science, 104: 1754-1761. 10.1002/app.25821 - Morais DS, Guedes RM, Lopes MA (2016) Antimicrobial Approaches for Textiles: From Research to Market.
Materials (Basel), 9(6): 498. doi: 10.3390/ma9060498 - Soares J, Moreira P, Queiroga A, Morgado J, Malcata F, Pintado M (2011) Application of immobilized enzyme technologies for the textile industry: A review. Biocatalysis and Biotransformation, 29: 223-237. doi: 10.3109/10242422.2011.635301
- González I, Oliver-Ortega H, Tarrés Q, Delgado-Aguilar M, Mutjé P, Andreu D (2017) Immobilization of antimicrobial peptides onto cellulose nanopaper. Int J Biol Macromol, 105(Pt 1): 741-748. doi:10.1016/j.ijbiomac.2017.07.094
- Alves D, Olívia Pereira M (2014) Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling, 30(4): 483-499. doi:10.1080/08927014.2014.889120
- Van Vliet K, Blouin V, Agbandje-McKenna M, Snyder RO (2006) Proteolytic mapping of the adeno-associated
virus capsid. Mol Ther, 14(6): 809-821. doi:10.1016/j.ymthe.2006.08.1222 - Lemon SM, Amphlett E, Sangar D (1991) Protease digestion of hepatitis A virus: disparate effects on capsid
proteins, antigenicity, and infectivity. J Virol, 65(10): 5636-5640. - Augustin M, Ali-Vehmas T, Atroshi F (2004) Assessment of enzymatic cleaning agents and disinfectants against bacterial biofilms. J. Pharm, Pharmaceut Sci, 7: 55e64.
- Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM (2013) Antimicrobial enzymes: an emerging strategy to
fight microbes and microbial biofilms. Biotechnol. J, 8: 97-109, https://doi.org/10.1002/biot.201200313 - Seabra IJ, Gil MH (2007) Cotton gauze bandage: a support for protease immobilization for use in biomedical
applications. Rev. Bras. Ciencias Farm, 43: 535-542. https://doi.org/10.1590/S1516-93322007000400006 - Shah Mahmud R, Müller C, Romanova Y, Mostafa A, Ulyanova, V, Pleschka S, Ilinskaya, O (2017) Ribonuclease
from Bacillus Acts as an Antiviral Agent against Negativeand Positive-Sense Single Stranded Human Respiratory RNA Viruses. BioMed Research International, 2017: 1–11. doi:10.1155/2017/5279065 - Minyu X, Joshua J, Jonathan G, Junjie C, Jiayi T, Ting L, Tieyi L, Joerg L, Zhan C (2018) Chemically immobilized antimicrobial peptide on polymer and self-assembled monolayer substrates. Langmuir, 34 (43): 12889-12896. doi: 10.1021/acs.langmuir.8b02377
- Laura A, Graça F, Claúdia M, Joana V, Isabel CG (2014) Bioactive microsphere-based coating for biomedicaltextiles with encapsulated antimicrobial peptides (AMPs). Ciência & Tecnologia dos Materiais, 26: 118–125. https://doi.org/10.1016/j.ctmat.2015.03.006
- Lang J, Yang N, Deng J, Liu K, Yang P, et al (2011) Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin
Binding to Heparan Sulfate Proteoglycans. PLoS ONE, 6(8): e23710. doi:10.1371/journal.pone.0023710 - van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK (2001) Antiviral activities of lactoferrin.
Antiviral Res, 52(3): 225-239. doi: 10.1016/s0166-3542(01)00195-4 - Jenssen H, Hancock RE (2009) Antimicrobial properties of lactoferrin. Biochimie, 91(1): 19-29. doi:10.1016/j.biochi.2008.05.015
- Orsi N (2004) The antimicrobial activity of lactoferrin: current status and perspectives. Biometals, 17(3): 189-
196. doi:10.1023/b:biom.0000027691.86757.e2 - Chen R, Cole N, Dutta D, Kumar N, Willcox MDP (2017) Antimicrobial activity of immobilized lactoferrin
and lactoferricin. J Biomed Mater Res B Appl Biomater, 105(8): 2612-2617. doi:10.1002/jbm.b.33804 - Han X, Yu Y, Wang Q, Fan X, Cui L (2014) Anti-bacterial properties of lactoferrin immobilized wool fabric. Indian Journal of Fibre and Textile Research, 39: 401-405.
- Małaczewska J, Kaczorek-Łukowska E, Wójcik R et al (2019) Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet Res, 15: 318. https://doi.org/10.1186/s12917-019-2067-6













