Article / Research Article
Dr. P-G Larsson
Department of Obstetrics and Gynecology
Skaraborgs hospital
SE-541 85 Skövde
Sweden
26 November 2020 ; 10 December 2020
To compare outcome of injection with the bulking agent polyacrylamide hydrogel (PAHG) with no treatment in women with urinary incontinence who were not candidates for treatment with a midurethral sling.
Women were randomized to treatment with PAHG or no treatment. After 2 months follow-up the women in the non-treatment group were also given PAHG treatment. All patients were then followed for 12 months. Patients were assessed with a patient satisfaction questionnaire, the UDI-6 (lower urinary tract symptoms) and IIQ-7 (quality of life). A new questioner was sent after 5 years.
At 2 months, IIQ-7 scores decreased by 55% and the UID-6 by 38% in women in the treated group compared with -4% and 2%, respectively in the non-treatment group. A total of 63% of patient were much satisfied/ satisfied in the treatment group compared with 19% in the non-treatment group. The 12-month follow-up showed a subjective satisfaction rate of 62%. The objective results show that women who were satisfied (n = 18) had a decrease in IIQ-7 of 61% and UID-6 of 41% compared with 20% and 10% in patients who were not satisfied (n = 11). At a mean (range) follow-up of 5 years (3–7), 44% of patients were still satisfied with treatment results.
Bulking treatment with PAHG can be offered to patients not suited to treatment
Keywords:Bulkamid, Mixed Incontinence, Stress Urinary Incontinence, Patient Satisfaction, Polyacrylamide Hydrogel
In Sweden, the first-line treatment option for stress urinary incontinence (SUI) has been the midurethral sling (MUS) using the tension free tape (TVT) [1]. However, certain women were deemed to be unable to undergo such a procedure due to contraindications for surgery or having previously received an MUS. There are very few therapeutic options for these women. Bulking agents have been used for in the treatment SUI for a number of years and have the benefit of being minimally invasive. Currently, bulking agents are recommended in the European Association of Urology guidelines for the treatment of uncomplicated SUI in women who would prefer a low-risk procedure, with the knowledge that repeat procedures may be required and long-term outcome has not determined [2]. A number of 12-month single arm studies have been reported showing favorable outcome [3-8].
The mode of action of the urethral bulking agent is through im¬proved coaptation of the urethra during the storage phase of the micturition cycle and particularly where abdominal pressure is increased. The bulking material is injected via periurethral or transurethral approaches, with the submucosa in the midurethral being target. Polyacrylamide hydrogel (PAHG; Bulkamid®) is a non-particulate bulking agent comprised of polyacrylamide hy-drogel (2.5% polyacrylamide and 97.5% water) and is non-biodegradable. The volume injected causes the bulking effect and a lasting network of fine fibers results from host cells entering the hydrogel, thus anchoring and perpetuating the bulking affect [9].
The purpose of this study is to provide treatment with a bulking agent to women who not eligible for treatment with a MUS.
From 2009, women with SUI or mixed incontinence at Skaraborgs Hospital in Skövde, Sweden were asked to take part in a prospective study where they would be randomized to treatment with PAHG or to no-treatment. Inclusion criteria were women with SUI or mixed incontinence who were ineligible for treatment with an MUS. Reasons being either a previous MUS procedure, no hypermobility of the urethra, a suspected low-pressure urethra or the existence of a co-morbidity such as cardiologic factors or significant obesity. Inclusion criteria included a micturition diary indicating a minimum of one voided urine of 250 ml or greater and a mean volume of 150 ml. Also required was a positive stress provocation test: (after coughing 10 times) and standing up from a chair 10 times with a minimum of 10 g of urine with minimum of 250 ml in the bladder or a 24-h pad test with more than 50 g of urine/24 h.
All women filled in the Urogenital Distress Inventory (UDI-6; lower urinary tract symptoms) and Incontinence Impact Questionnaire short form (IIQ-7; quality of life) questionnaires. The urethral resistant pressure (URP) was also measured in a method described by Slack et al. [10-12]. The normal value for URP is 74 cmH2O and in the case of SUI, 55 cmH2O [13].
The procedures were conducted on an outpatient basis and took approximately 15 min under endoscopic control under intraurethral anesthesia (2 ml 5% lidocaine injected into the urethra). The procedure has previously been describe [14]. Following bladder emptying, PAHG was injected under urethroscopy using a special urethroscope (Bulkamid® Urethral Bulking System) with a 0 degree optical lens. The injections are done transurethrally into the submucosa (three deposits of 0.2–0.8 ml each 0.5–1.0 cm distal from the bladder neck at 2, 6 and 10 o’clock using a 23-gauge needle with 1 cm markings to ensure correct depth of injection). To ensure that the urethra was not obstructed, NaCl was flushed into the bladder. Following injection, patients were asked to cough and if leakage occurred an additional injection was carried out. Patient were discharged after voiding with a documented residual urine of less than 100 ml.
All patients were followed up after 2 months using the stress provocation test as described above. The same UDI-6 and IIQ-7 questionnaires were used to evaluate treatment. Patients were also asked about the overall subjective response, which was divided into four categories: much satisfied, satisfied, same, and worse.
The women randomized to no-treatment were then offered treatment with PAHG and then followed at 2 months. For all patients who were not completely dry at 2 months, a top up treatment was performed. At 12-month post-treatment, women were again asked to do a stress provocation test and to complete the IIQ-7 and UDI-6 questionnaire. Patents who were satisfied at the 12 months follow up were then followed up by an additional overall subjective response, and a single question regarding whether the patient would recommend the treatment. Women were also followed using the tool provided by the Swedish National Quality Register of Gynecological Surgery (a.k.a GynOp) at 2 months and 12 months. This assesses subjective cure.
The study was approved by the regional ethical committee in Gothenburg and Stockholm. Number Dnr 008-09 approved 22 June 2009 and Clinical trial registered 24 Sept 2009 # NCT00984958.
A total of 32 patients were recruited at Skaraborgs Hospital in Skövde. Other patients studied under the same protocol, not at Skövde are not included. Sixteen patients were randomized to treatment with PAHG and 16 were randomized to no treatment (Fig 1).
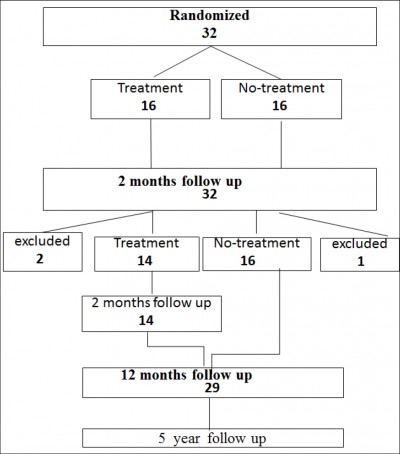 Figure 1: Study flow chart
Figure 1: Study flow chart
The mean (range) age was 68 (35–89) years in the treatment group and 74 (55–88) years in the expectance group. The most common reason for unsuitability for a MUS procedure was a previous TVT procedure (Table 1).
| PAHG treatment group | No treatment group | |
|---|---|---|
| Previous TVT procedure | 9 | 8 |
| Previous Burch colposuspension |
0 | 2 |
| Other bulking agents | 0 | 3 |
| ISD | 1 | 2 |
| High BMI; age | 4 | 1 |
| Vaginal mesh operation | 2 | 0 |
| Mixed incontinence | 14 | 16 |
| Stress incontinence | 2 | 0 |
Table 1: Patient characteristics making them unsuited to treatment with a transvaginal tape. BMI > 60 and age above 90.
TVT : tensionfree vagina tape;
ISD : internal sphincter deficiency;
BMI : body mass index
Two women had been operated with vaginal anterior mesh and they did not have any hypermobile urethra. All women had mixed incontinence except two women, one with vaginal anterior mesh and one who had undergone two MUS procedures. Of the other women, three had a diagnosis of internal sphincter deficiency (ISD) and five had medical reason for not undergoing any surgery due to other diseases or to a high BMI.
One patient was treated outside the protocol due to her aggressive ovarian cancer. She presented with increasing urinary leakage when she was standing up. She had a positive cough test during examination, but she had also her third relapse of ovarian cancer with ascites and a mass in the vagina. She was offered PAHG without randomization because her expectant survival was very short. She was very happy for the bulking agent as she was dry for 3 months. During this time, she also had treatment with cytostatic drugs. She scored the improvement in quality of life due to the bulking as especially notable in the context of high cost chemotherapy.
Patient characteristic at baseline and at 2-months follow-up are shown in Table 2.
| Treatment (n = 16) | No treatment (n = 16) | |||||
|---|---|---|---|---|---|---|
| Parameter Mean + (SD) |
Baseline | 2 months | difference | Baseline | 2 months | difference |
| IIQ-7 (SD) | 51 (19.6) | 23 (24.5) | 55% | 52 (20.7) | 54 (24.6) | -4% |
| UID-6 (SD) | 58 (12.1) | 36 (18.8) | 38% | 62 (12.3) | 61 (16.1) | 2% |
| leakage (g) (SD) | 31 (21.6) | 2.5 (2.9) | 92% | 53 (49.2) | 64 (61.7) | -21% |
| URP (SD) | 29.8 (9.2) n = 13 |
29.2 (7.8) n = 12 |
||||
| Subjective results | ||||||
| much satisfied | 2 | 63% | 0 | 19% | ||
| satisfied | 8 | 3 | ||||
| same | 5 | 9 | ||||
| worse | 1 | 4 | ||||
Table 2: Patient characteristics at baseline and 2 month follow-up
IIQ-7 : Incontinence Impact Questionnaire short form;
UID-6 : The Urogenital Distress Inventory;
URP : urethral resistance pressure
Among the women who were treated, the IIQ-7 decreased by 55% and the UID-6 by 38% in contrast to -4% and 2% in the untreated group. The mean urine leakage was reduced by 92% in the treated group compared with an increase of 21% in the untreated group. In the treated group, 63% of patients reported feeling much satisfied/satisfied compared with 19% on the no treatment group.
At 2 months the no-treatment group were offered the same bulking treatment as the original treatment group – all had continuing urinary incontinence. Of these 16 patients, two patients were excluded and not treated; one due to pancreatic cancer and the other had surgery for malignant ascites. One patient was also excluded from the treatment group because she was operated for pelvic organ prolapse.
Follow-up at 12 months post-treatment was available in 29 patients (Table 3).
| Satisfied (n = 18) | Not satisfied (n = 11) | |||||
|---|---|---|---|---|---|---|
| Parameter Mean + (SD) |
Baseline | 12 months | % difference | Baseline | 12 months | % difference |
| Mean IIQ-7 (SD) | 51 (19.5) | 20 (19.9) | 61% | 54 (21.7) | 43 (25.8) | 20% |
| Mean UID-6 (SD) | 59 (13.0) | 35 (19.5) | 41% | 61 (11.9) | 55 (19.1) | 10% |
| Mean leakage (g)(SD) | 31 (30.9) | 6 (14.7) | 81% | 64 (47.5) | 60 (43.2) | 6% |
| Subjective results | ||||||
| much satisfied | 3 | 62% | 0 | 38% | ||
| satisfied | 15 | 0 | ||||
| same | 0 | 8 | ||||
| worse | 0 | 3 | ||||
Table 3: Subjective and objective results at 12 months follow-up in patients treated with PAHG.
IIQ-7 : Incontinence Impact Questionnaire short form;
UID-6 : The Urogenital Distress Inventory
Eighteen (62%) of the 29 patients were much satisfied/ satisfied with treatment with the remaining 11 patients (38%) the same or worse. In patients who were satisfied with treatment, mean IIQ-7 scores were decreased by 61% compared with 20% in the unsatisfied group. Similarly for mean UID-6 scores were the equivalent values were 41% and 10%. Mean urinary leakage was reduced from baseline with 81% and 6% in the satisfied and unsatisfied groups, respectively.
The 18 women who were satisfied at the 12 months were approached to complete the additional questionnaire at a mean (range) follow-up of 5 (3–7) years (Fig 2). Of these, one patient had died, and one did not answer the questionnaire providing results in 16 women. Seven (44%) patients were very satisfied or satisfied; and they scored a decrease from the original IIQ-7 and UDI 6 with 76% and 69%. Of the nine women with no or worse effect the change in IIQ-7 and UDI from the original was an increase in 44% and 15% respectively. Eight women would have liked to have new treatment and 11 would recommend the PAHG treatment to other women. Among the women who were satisfied, three were receiving treatment for overactive bladder. Among the women showing no benefit of PAHG treatment, a lot of comorbidity had occurred. One had severe chronic obstructive pulmonary disease, two had had a stroke, one interstitial cystitis, one rectal cancer with radiotherapy, one was treated successfully with Botox and one had severe Parkinson’s disease. The last one had before the PAHG injection a MUS with little effect and she wanted to try a new injection with PAHG.
Reported complications following treatment were minimal, with one patient being unable to void sufficiently and requiring intermittent catheterization for one night.
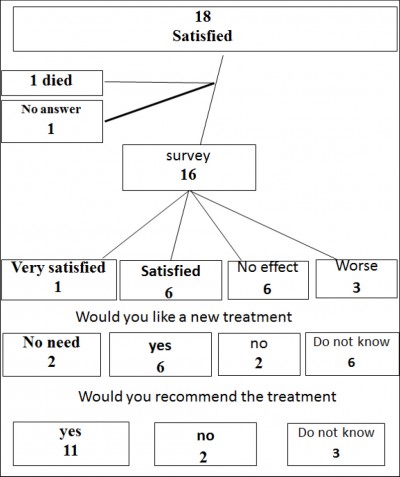
Figure 2: The 5 year questionnaire results
This was the first randomized study comparing treatment with PAHG to no treatment. Results showed a subjective improvement rate of 63% was achieved at 2 months following injection of PAHG and this rate was maintained at 12 months.
These data compare with 19% at 2 months in the untreated patient group.
At a mean follow-up of 5 years, 44% of patients were still satisfied with the treatment. These results compare with previous single arm studies of PAHG, which reported subjective response rates (cured or improved) at 12 months of 48–86.6% [3-8].
The subjective satisfaction rates show similar results to the objective results with IIQ-7 and UDI 6. Among women who report satisfaction (at 2 months) a mean decrease in IIQ-7 and UDI 6 was 55% and 38% respectively. At the 12 months follow if the women were satisfied she scored a decrease of the IIQ-7 and UDI 6 score of 61% and 41% respectively. This will still be the same at the 5 year follow up were the satisfied scored a decrease of 76% and 61%. In the not satisfied group small or no changes and even increase in the IIQ-7 and UDI 6 score. The IIQ-7 and UDI 6 will be a good measure of the subjective result of an incontinence.
The URP data in the current study shows that all patients had low pressure (< 30 cmH2O), suggesting the presence of intrinsic sphincter deficiency. Unfortunately, the instrument was withdrawn from the market so no follow-up data were available. Treatment complications in the study were low and compare favourably to previous studies [3-8].
Studies specifically in older patients show similar results to those reported in the current study [15,16]. Patients in this group have sufficient symptoms to need medical care either by the general practitioners or urotherapist as well as incontinence pads, all at a continuing cost. An efficient and safe treatment of incontinence in this patient group would be of value. A 5-year follow-up in this elderly population shows also that the co-morbidity is high with the new onset of new diseases that also have an impact on incontinence [17].
PAHG injection could be offered to women not cured after a MUS procedure and be expected to lead to an increase the quality of life of these women [18]. In one study, women with SUI and mix incontinence were treated with a MUS and if not successful they were offered a treatment with PAHG [19]. More recently, a randomized trial with the opposite approached has been published. Women were randomized to PAHG injection or MUS treatment. Subjective satisfaction was reported in 80% and 100% in the bulking and MUS groups, respectively [20]. Among the women in the PAHG group, 18 of the 113 patients in the PAHG group went on to receive a MUS. Of note, the majority of perioperative complications and reoperations due to complications were associated with the MUS.
One drawback of the study is the small number of patients reported here. The original protocol was intended for a larger cohort and this current publication focuses on the patients from a single center.
It can be concluded that bulking treatment with PAHG provides satisfactory objective and subjective outcomes for up to 5 years in women who are not eligible for treatment with a MUS.
- Swedish National Quality Register of Gynecological Surgery (a.k.a GynOp). http://www2.gynop.se/home/ about-gynop/; accessed 20 December 2020.
- Burkhard FC, Bosch JLHR, Cruz F, Lemack GE, Nambiar AK, et al. European Association of Urology Guidelines on Uronary Incontinence.
- Lose G, Mouritsen L, Nielsen JB (2006) A new bulking agent (polyacrylamide hydrogel) for treating stress urinary incontinence in women. BJU Int 98: 100-104.
- Lose G, Sørensen HC, Axelsen SM, Falconer C, Lobodasch K, et al. (2010) An open multicenter study of polyacrylamide hydrogel (Bulkamid®) for female stress and mixed urinary incontinence. Int Urogynecol J 21: 1417- 1471.
- Leone Roberti Maggiore U, Alessandri F, Medica M, Gabelli M, Venturini PL, et al. (2013) Outpatient periurethral injections of polyacrylamide hydrogel for the treatment of female stress urinary incontinence: effectiveness and safety. Arch Gynecol Obstet 288: 131-137.
- Sokol ER, Karram MM, Dmochowski R (2014) Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol 192: 843-849.
- Zivanovic I, Rautenberg O, Lobodasch K, von Bünau G, Walser C, (2017) Urethral bulking for recurrent stress urinary incontinence after midurethral sling failure. Neurourol Urodyn 36: 722-726.
- Krhut J, Martan A, Jurakova M, Nemec D, Masata J, et al. (2016) Treatment of stress urinary incontinence using polyacrylamide hydrogel in women after radiotherapy: 1-year follow-up. Int Urogynecol J 27: 301-305.
- Christensen LH, Nielsen JB, Mouritsen L, Sørensen M, Lose G (2008) Tissue integration of polyacrylamide hydrogel: an experimental study of periurethral, perivesical, and mammary gland tissue in the pig. Dermatol Surg 34: S68-77.
- UID-6 Urogenital distress inventory. https://www.ohsu. edu/sites/default/files/2019-06/Female-Urology-Questionnaire-6.pdf.
- IIQ-7 incontinence impact questionnaire -short form. http://www.gericareonline.net/tools/eng/urinary/attachments/UI_Tool_2_IIQ7_SF.pdf.
- Slack M, Culligan P, Tracey M, Hunsicker K, Patel B, et al. (2004) Relationship of urethral retro-resistance pressure to urodynamic meas-urements and incontinence severity. Neurourol Urodyn 23: 109-114.
- Digesu GA, Athanasiou S, Chaliha C, Michalas S, Salvatore S, et al. (2006) Urethral retro-resistance pressure and urodynamic diagnoses in women with lower urinary tract symptoms. BJOG 113: 34-38.
- Kasi AD, Pergialiotis V, Perrea DN, Khunda A, Doumouchtsis SK (2016) Polyacrylamide hydrogel (Bulkamid(R)) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J 27: 367-375.
- Hillary CJ, Osman N, Chapple C (2015) Considerations in the modern management of stress urinary incontinence resulting from intrinsic sphincter deficiency. World J Urol 33: 1251-1256.
- Vecchioli-Scaldazza CV, Smaali C, Morosetti C, Azizi B, Giannubilo W, et al. (2014) Polyacrylamide hydrogel (bulkamid(R)) in female patients of 80 or more years with urinary incontinence. Int Braz J Urol 40: 37-34.
- Pai A, Al-Singary W (2015) Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid(®) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent European J Urol 68: 428-433.
- Martan A, Masata J, Svabik K, Krhut J (2014) Transurethral injection of polyacrylamide hydrogel (Bulkamid®) for the treatment of female stress or mixed urinary incontinence. Eur J Obstet Gynecol Reprod Biol 178: 199-202.
- Zivanovic I, Rautenberg O, Lobodasch K, von Bünau G, Walser C, et al. (2016). Urethral bulking for recurrent stress urinary incontinence after midurethral sling failure. Neurourol Urodyn 36: 772-776.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, Tulokas S, Mikkola TS (2020) TVT surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence : a randomized clinical trial. J Urol 203: 372-378.













