Article / Research Article
1University of Groningen, Netherlands.
2Federico II University of Naples, Italy.
Justin Bulten
University of Groningen,
Netherlands.
20 February 2024 ; 14 March 2024
The peroxide degradation of polypropylene was studied in supercritical carbon dioxide (scCO2) in the presence of a N-hydroxyphthalimide (NHPI). Six levels of NHPI concentration and 6 levels of peroxide concentration were selected and each permutation was tested both with and without scCO2. It was observed that the NHPI would increase degradation at lower peroxide concentrations (<0.1 wt. %), but would suppress degradation at higher peroxide concentrations (>0.2 wt. %). Furthermore, it was discovered that at an NHPI concentration of 3.3 wt. %, the stereo regularity slightly decreased with increasing peroxide concentrations.
Keywords: Polypropylene, degradation, β-scission, epimerization, peroxide, n-hydroxyphtalimide, supercritical carbon dioxide.
During the last 3 decades, N-hydroxyphthalimide (NHPI) has been receiving a moderate amount of attention in literature, with particular interest into its function as organic catalyst for oxidation reactions (Hara et al., 2001; Liang & Jiao, 2017; Lin et al., 2012; Matsunaka et al., 1999; Minisci et al., 2004; Mo & Jensen, 2018; Opeida et al., 2011; Opeida et al., 2019). The operating mechanism is to generate the phthalimide-N-oxyl radical (PINO) from NHPI. As was reported in 2019, unlike other nitroxides such as TEMPO, PINO does not partake in reversible combination of radicals, but rather propagates the reaction scheme by abstracting a hydrogen elsewhere, and subsequently revert into NHPI.
However, that leaves the question of how it is initiated. Some reports involve a co-catalyst, e.g. cobalt or manganese, (Koshino et al., 2003; Minisci et al., 2004) and some claim that temperature and oxygen have an effect (Lin et al., 2012). Obviously the question becomes obsolete if a radical initiator is used.
PINO can abstract a hydrogen from both secondary and tertiary carbon atoms, but the tertiary carbon atom has a predominantly higher selectivity (Matsunaka et al., 1999). Presumably this is because of a higher effect of stabilization due to the inductive effect of electron donating groups surrounding this tertiary carbon.
Next to oxidation, there have also been reports on forming C-N bonds by reacting an organic radical with azodicarboxylates (Amaoka et al., 2012; Chen et al., 2021; Zhou et al., 2017; Zhou et al., 2019). In this process, NHPI seems to play the exact same role as for the oxidation processes: catalytic hydrogen abstraction. In this respect, NHPI plays a remarkably similar role to other radical initiators such as peroxides; however, with the clear distinction that the hydrogen abstraction by PINO is reversible, making it a catalyst, whereas hydrogen abstraction by peroxides is not reversible, thus requiring stoichiometric amounts. The catalytic nature of NHPI gives it a clear advantage over alternative radical initiators.
NHPI has even been immobilised on polymer substrates, in order to open up the possibility of heterogeneous catalysis, thus eradicating the need to separate NHPI from the reaction mixture afterwards (Culica et al., 2019; Gao et al., 2015; Jian et al., 2016). However, having a catalyst immobilized on a polymer might be an option for low molecular weight systems in solution, but will not be as ideal for polymer melt processes.
Still, these functionalization reactions were performed with the more electronegative heteroatoms nitrogen and oxygen, while it has also been reported that C-C bonds can be formed. In another work by Opeida, methyl methacrylate was polymerized using NHPI as initiator (Opeida et al., 2011). Moreover, NHPI was discovered to play another role in that process: namely as stereoregulator. By forming hydrogen bonds with the ester groups of the methacrylates, NHPI was supposed to increase the size of the side group of the polymers backbone to such a degree that steric hindrance prevented for the next unit to follow the same stereochemistry as the unit before, thus resulting in a syndiotactic chain (Gao et al., 2015).
Aside from initiating and guiding the polymerization process itself, NHPI can also be employed as an organic catalyst for post-polymerization modification of polyolefins. Both Zhou and Chen reported successful metal-free amination of polyethylene (PE) and polypropylene (PP) (Chen et al., 2021, Zhou et al., 2017, Zhou et al., 2019). The latter is particularly interesting as radical reactions on PP are mostly difficult to control; however, in both cases this was done in the solid state, at 80°C and 100°C. Indeed, the propensity of PP to undergo β-scission during radical reactions is well documented. At higher temperatures, β-scission becomes quite prevalent, which is why melt processes often display a significant component of degradation. In fact, β-scission occurs so fast that most modelling studies assume it happens instantaneously, and as a consequence its kinetic constant is often embedded in the other reactions such as H-abstraction. On the other hand, the PP macro radical tends to be slightly more stable at lower temperatures, which is why long-chain branching of PP is also performed in the solid state, namely by irradiation (Lee et al., 2013), Scheve et al., 1990, Scheve et al., 1998). Nevertheless, long-chain branching has also been achieved in the melt by employing coupling agents. This would suggest that there are parallel processes that run sufficiently fast to offer serious (i.e. with comparable kinetics) competition to β-scission. Additionally, by employing techniques from controlled radical polymerization, various post-polymerization processes of PP are possible without significant degradation (Coiai et al., 2010; Coiai et al.,2019; Passaglia et al., 2009).
As such, virtually every melt process with PP becomes a “kinetic race” where β-scission is always relevant. Any desirable follow-up reaction is virtually always mass-transfer limited; therefore, a plasticizer can be utilized in order to help overcome the mass-transfer limitation. Properties such as viscosity of, solubility in, and diffusivity through the melt are improved in the presence of a plasticizer. One such plasticizer that has been getting more and more attention is supercritical CO2 (scCO2).
Like any supercritical fluid, scCO2 exhibits hybrid properties of the classical gas and liquid phase; therefore, it can boost its properties using both the solubility that is typical of liquids, and the diffusivity that is typical of gases. Combining the best features of both phases, scCO2 might prove a valuable asset in supporting any desirable follow-up reaction by improving the mass-transfer rate (Nalawade et al., 2006; Picchioni, 2014). CO2 is particularly interesting because its critical point is relatively mild (74 bar, 31°C). Besides, CO2 is benign, non-toxic, and relatively inert. This makes it an ideal substance for green chemistry [ref principles of green chemistry by ACS].
Nevertheless, β-scission offers up some advantages in the form of narrowing the traditionally wide polydispersity index (PDI) of Ziegler-Natta catalysed PP. A narrower PDI yields interesting rheological and mechanical properties, which is why β-scission has been actively pursued to produce so called Controlled Rheology PP (CR-PP). However, such processes usually involve substantial quantities of peroxides, which is why the catalytic scheme of NHPI is of interest (Iedema et al., 2011; Ryu et al., 1991a; Ryu et al., 1991b; Tzoganakis et al., 1988; Tzoganakis et al., 1989; Vergnes & Berzin, 2000).
It is not new to consider nitroxides as alternative radical initiators to peroxides (Pfaendner, 2006) If only as a safety precaution, because nitroxide can act as flame-retardants, some of which already at concentrations as low as 0.5wt.% (Pfaendner, 2006) Regardless, even though tailor-made nitroxides have been demonstrated to be more effective chain scissors, the fact that they are tailor made means that they are not yet suitable for commercial application (Pfaendner, 2006). However, if β-scission is the desired effect, these compounds become quite relevant (Minisci et al., 2004).
As mentioned before, β-scission prevails in melt processes such as reactive extrusion. At the temperatures required for PP to be in the melt phase, the kinetics favour degradation. At lower temperatures, the rate of β-scission is lower to the extent that it won’t always dominate the reaction scheme and other reactions can become more prevalent (Borsig et al., 2001; Borsig et al., 2008; Liu et al., 2005; Rätzsch et al., 2002). However, due to the higher melting temperature of PP, reactions at low temperature are necessarily either in solid state, or in solution; though even in solution, the temperature needs to be at least 100°C, and probably 125°C, before PP dissolves.
Solid-state reactions are mass transfer impaired, due to virtually no chain mobility. The amorphous phase may be sufficiently porous for coagents to diffuse, but the crystalline phase is not capable of absorbing such coagents because it is too densely packed. Hence, the preference in this project for melt processes, because the result can be more uniform, rather than only affecting the amorphous phase and leaving the crystalline phase unchanged.
This work investigates the effect of NHPI on polypropylene during reactive extrusion. A number of mixing ratios were tested, both with and without the presence of scCO2 and their results are discussed. Particular attention was paid to the effect on molecular weight to check on the effect of β-scission and epimerization, as well as side reactions, such as grafting and branching.
Materials
Polypropylene PP571P was used as supplied by Sabic, N-hydroxyphthalimide (NHPI, 98%) was used as supplied by Sigma-Aldrich, and 2,5-Dimethyl-2,5 (tert-butylperoxy) hexane peroxide (Trigonox 101) was used as supplied by Nouryon.
Reactive extrusion experiments were carried out in a Three-Tec extruder (Ø 12 mm, L/D 25) with 5 barrel zones excluding die and feeding zone. The first sector was kept at 140°C, the remaining 4 were all set to 200°C. The throughput rate was 180 g/h and the speed was set to 30 RPM, which yields an average residence time of 80 seconds. The half-life time of the peroxide at 200°C was calculated to be 6.25 seconds using the kinetic data from the brochure (Nouryon, n.d.).
The PP pellets were mixed with the NHPI powder and the peroxide droplets, and the mixture was fed to the hopper. A combination of 6 levels of peroxide concentrations, 6 levels of NHPI concentrations, where they were tested both in the presence of 4 wt.% scCO2 and without it, which adds up to a total of 72 experiments. Table 1 shows the parameters for each concentration level of peroxide and NHPI. The experiments without scCO2 are denoted as Nxy, those with scCO2 are denoted as Cxy, where x and y represent row and column, thus Nxy and Cxy refer to the position in Table 1.
The peroxide concentration is formatted as active oxygen using this formula:
C X 0,1014,
Where C is the peroxide concentration in PPM
| NHPI (mg/g PP) | NHPI (mg/g PP) | |||||
|---|---|---|---|---|---|---|
| 0 | 10 | 50 | 100 | 200 | 1000 | |
| 0 | N00/C00 | N01/C01 | N02/C02 | N03/C03 | N04/C04 | N05/C05 |
| 0.33 | N10/C10 | N11/C11 | N12/C12 | N13/C13 | N14/C14 | N15/C15 |
| 1.6 | N20/C20 | N21/C21 | N22/C22 | N23/C23 | N24/C24 | N25/C25 |
| 3.3 | N30/C30 | N31/C31 | N32/C32 | N33/C33 | N34/C34 | N35/C35 |
| 6.7 | N40/C40 | N41/C41 | N42/C42 | N43/C43 | N44/C44 | N45/C45 |
| 33.3 | N50/C50 | N51/C51 | N52/C52 | N53/C53 | N54/C54 | N55/C55 |
The extrudate was collected in chunks of about 1-2 cm. These chunks were ground into smaller ones of 1-4 mm using a MO-DI-TEC MINI BM. These smaller pellets were washed with acetone for 6 hours using a Foss Soxtec 2043 and dried overnight to remove low molecular weight impurities.
MFI: The melt flow index (MFI) was measured in a Ceast MF20 from Instron, using standard ASTM D1238, at 230°C and 2.16 kg. About 4-5 grams were inserted and preheated for 7 minutes to melt without load while the discharge die was blocked to prevent any flow during this preheating time. The die was opened at the end of the preheating time, after which followed a preheating interval with load, until a height of 46 mm was reached, and the measurement of 30 mm was started.
Rheology: The rheological frequency sweep was performed from 100 rad/s to 0.1 rad/s at 180°C to plot the viscosity over shear rate using a HR-2 Discovery Hybrid Rheometer by TA Instruments. The discs were melted and slightly pressed to ensure that the parallel plates were fully covered. Then a preheating time of 5 minutes followed to soak the discs. Then a Dynamic Strain Sweep (DSS) was performed at 100 rad/s to select the strain for the Dynamic Frequency Sweep (DFS) which was measured at the selected strain from 100 rad/s to 0.1 rad/s. The DFS was fitted using the Yasuda-Carreau model (Seavey et al., 2003; Wu et al., 2023).
A time sweep was performed for one sample as a check for stability over time. This was done at 250°C at 3.14 rad/s for 180 seconds. This step was performed after a DSS, which covered a preheating time of 5 minutes.
FTIR: FTIR was performed using a IRTracer-100 from Shimadzu.
DSC: Differential scanning calorimetry (DSC) was performed in a DSC 25 from TA instruments. The sample is first heated to 230°C at 10 °C/ min and kept there for 5 minutes for the sample to completely melt so as to erase the thermal history. Then the sample is cooled to -50°C C at 10 °C/ min, and subsequently heated to 230°C at 10 °C/ min.
NMR: Quantitative spectra were recorded using a Bruker Advance III 400 spectrometer equipped with a high-temperature cryoprobe for 5 mm OD tubes and a robotic sample changer with preheated carousel (24 positions), on 45 mg mL-1 polymer solutions in tetrachloroethane-1,2-d2 (with BHT added as a stabilizer, [BHT] = 0.4 mg mL-1). Acquisition conditions for 13C NMR were: 45° pulse; acquisition time, 2.7 s; relaxation delay, 3.3 s; 2 K transients. Broad-band proton decoupling was achieved with a modified WALTZ16 sequence (BI_WALTZ16_32 by Bruker). The concentration of stereodefects in the isotactic fraction was obtained from the 13C NMR spectra of raw samples, by measuring the fractional amount of the mmmrrmmm nonad in the methyl region (Busico et al., 2016; Busico & Cipullo, 2001).
Degradation
In 1996, Gahleitner et al published a reasonably good correlation between Mw and MFI for PP (Gahleitner et al., 1996). The PDI seems to have somewhat of an influence, which is why the correlation forms more of a band rather than a line. Previous work has gathered data that nicely fits within that band, thus corroborating the correlation (Figure 1). This can be used to make a reasonable estimate of the Mw of the samples by measuring the MFI. As such, the MFI can give an excellent indication of the degree of degradation. An increasing MFI relates to a reduction in molecular weight as a result of degradation; the higher the MFI, the higher the extent of degradation.
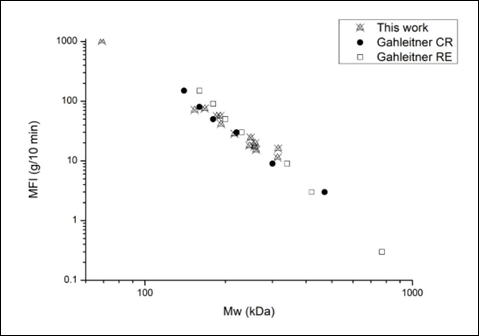 Figure 1: Correlation between MFI and Mw ●CR = controlled rheology PP □RE = reactor grade PP
Figure 1: Correlation between MFI and Mw ●CR = controlled rheology PP □RE = reactor grade PP
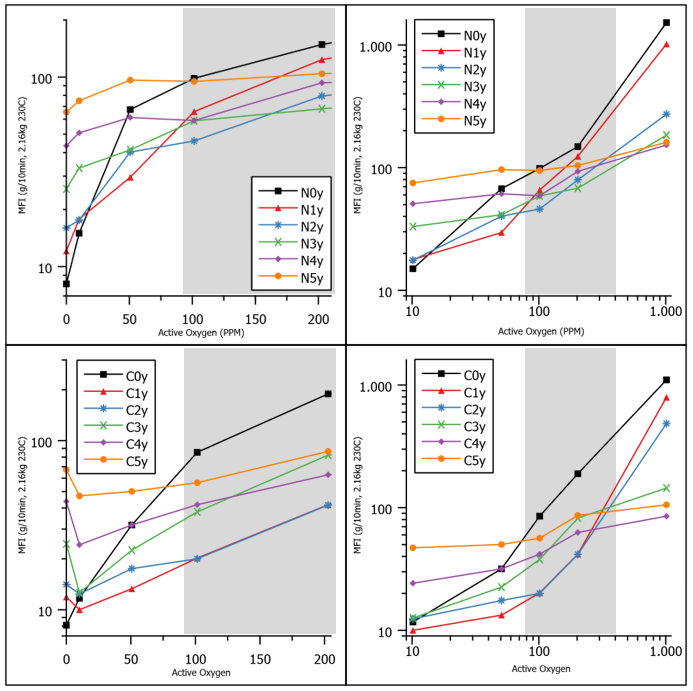 Figure 2: MFI as function of active oxygen. Linear scale (left), log scale (right), without scCO2 (top), and with scCO2 (down). N/C represents the experiments without and with scCO2, x represents the amount of NHPI, and y represents the amount of peroxide (see Table 1 for values).
Figure 2: MFI as function of active oxygen. Linear scale (left), log scale (right), without scCO2 (top), and with scCO2 (down). N/C represents the experiments without and with scCO2, x represents the amount of NHPI, and y represents the amount of peroxide (see Table 1 for values).
The MFI data obtained shows a very clear pattern (Figure 2). At low active oxygen, increasing NHPI leads to increasing MFI. At high active oxygen, the reverse is true: increasing amounts of NHPI reduces the MFI increase. At intermediary levels of active oxygen, there is a cross-over region (roughly from 80-400 PPM, shaded in grey). This trend is observed for the case with scCO2, as well as the case without it; however, the MFI seems to be lower across the board with scCO2 compared to the case without it. Although, the exception to that statement is found on the vertical axis, where the experiments without peroxide are represented. When scCO2 is used, the experiments with only NHPI are virtually the same as when scCO2 is not used. This would suggest that the effect of NHPI is not hindered, whereas the CO2 seems to be an obstacle to the peroxide to some extent.
Interestingly, with the second highest amount of NHPI, the MFI is about as low or even lower than with the highest amount used, hinting at some sort of optimum. When the amount is increased even further, the following can be observed, confirming the optimum trend (Figure 3).
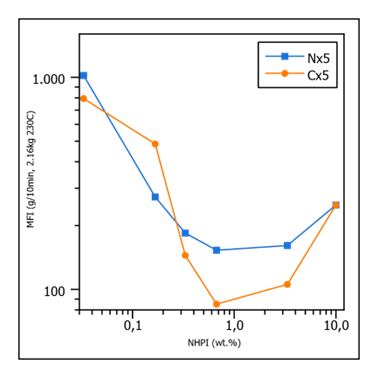 Figure 3: MFI at 1 wt% peroxide as function of NHPI content
Figure 3: MFI at 1 wt% peroxide as function of NHPI content
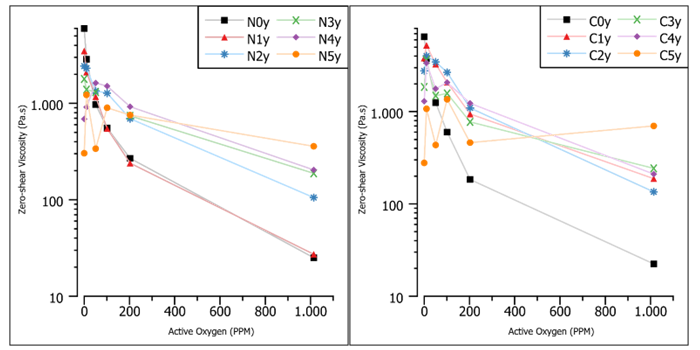 Figure 4: Zero shear viscosity (extrapolated from DFS)
Figure 4: Zero shear viscosity (extrapolated from DFS)
The rheology data corroborates this trend (Figure 4). At low active oxygen, the zero shear viscosity is lower as more NHPI is added, indicating a lower MW. At high active oxygen, the order is reversed, and the samples with the most NHPI have the higher zero-shear viscosity, indicating a higher MW. By using Figures 1-4, a couple of observations will be discussed to construct the mechanism (Scheme 1).
Scheme 1 provides a summary of all possible and relevant reactions, part of which is based on literature (Berzin et al., 2000; Iedema et al., 2001; Tzoganakis et al., 1989). The mechanism starts by the initiator decomposing into two radical species (1). These radicals abstract a hydrogen from the PP backbone (3a), which usually undergoes -scission (4). -scission does not terminate the radicals, this is done through disproportionation (8a) or recombination (8b). However, before terminating, the radicals can propagate via chain transfer, either before -scission (5a) or after (5b).
In the absence of NHPI, the system shows the traditional characteristics of peroxide degradation of PP. The MFI rises with increasing active oxygen, which is a metric of the radical concentration in the reaction mixture.
Clearly, the presence of NHPI interferes with the usual workflow of the peroxide to degrade the polymer. At high active oxygen (Nx5/ Cx5), the more NHPI is added, the lower the MFI turns out to be. If NHPI had no effect on the peroxide, it would be expected that the MFI to be at least as high as when it is absent and only peroxide is used. This is not the case; NHPI mitigates degradation.
At low active oxygen (Nx1/Cx1), the trend is reversed; the MFI increases rather than decreases when more NHPI is added. Therefore, NHPI facilitates degradation of PP. Indeed, without the use of peroxide, increasing amounts of NHPI also lead to a higher MFI, thus indication more degradation the more it is used. A stark contrast with its behaviour at high active oxygen. NHPI seems to be capable of both mitigating and facilitating degradation, depending on the active oxygen concentration.
As mentioned in the introduction, NHPI has been used as catalyst to oxidize organic compounds through a cycle of PINO generation and H-abstraction. Assuming PINO also abstracts a hydrogen from the PP backbone, degradation would follow through β-scission. This scenario is similar to the case of peroxide degradation, with the only exception that some other molecule abstracts the hydrogen. The macroradical still breaks through a unimolecular mechanism.
NHPI is able to facilitate degradation, but not nearly as effectively as Trigonox 101. At 6 times the molar amount, NHPI raises the MFI to about 65, whereas Trigonox goes to about 1600. As such, Trigonox has roughly 25 times more effect with 1/6 the amount. Furthermore, NHPI has regenerability into PINO, and so NHPI works catalytically, whereas Trigonox works stoichiometrically. Part of the explanation may be a different rate of PINO generation compared to the thermal decomposition of the peroxide (Scheme 1, 2b). Another explanation could be a different rate in hydrogen abstraction (Scheme 1, 3b). The latter would also explain the mitigating effect at high active oxygen concentrations.
 Scheme 1: Complete overview
Scheme 1: Complete overview
It seems likely based on the data in that PINO plays a role in the mechanism. However, given that NHPI is added and not PINO, the latter has to be generated from the former. This can be done in a variety of ways, as exemplified in reactions (2) in Scheme 1.
Reaction (2a) generates PINO through the peroxide radical. The mitigating effect at high peroxide concentrations is a clear indication that NHPI interferes with the peroxide radical. However, it is not likely the only pathway to generate PINO, since the MFI keeps increasing with increasing amounts of NHPI in the absence of a peroxide.
Therefore, reaction (2b) presents a second pathway through a thermal trigger since no other coagents are present. Therefore, none of the proposed mechanisms throughout literature applies to this investigation (Coseri, 2009). In this situation, only heat and shear stress are applicable.
To propagate the catalytic cycle, PINO has to be regenerated, which may be done by returning a hydrogen atom to the macro radical before it has undergone β-scission, as reflected in reaction (2c). Reaction (2d) shows that the same may be possible after the macro radical has undergone β-scission. This would infuse an intermediary step between β-scission and the ensuing secondary radical abstracting a hydrogen from another PP chain to propagate the degradation mechanism. In other words, having reactions (2d) and (3b) form a substitute for reaction (5b).
Still, it could be that the system was not entirely air-tight, and some unwanted oxygen molecules could have entered the extruder and generated PINO from NHPI by taking the hydrogen, much like what was mentioned in the intro. However, FTIR does not show the presence of a carbonyl peak (Figure 6), so there is no evidence that the PP chains have actually been oxidized through such a scheme. This would leave this postulation questionable. Additionally, it is not necessary to postulate it to begin with, as plenty of alternatives are available.
Since the exact same temperatures are used, the half-life time of the peroxide is the same in each instance; therefore, the same number of radicals would be expected. The increasing amount of NHPI added would make it increasingly probable for a peroxide radical to react with an NHPI molecule instead of the polymer. In other words, reaction (2a) becomes more predominant relative to reaction (3a) compared to situations where the NHPI concentration is lower. This would shift the RO/PINO ratio to relatively more PINO.
Note, however, that PINO is itself a radical, and so the total number of radicals isn’t reduced, whereas the MFI is reduced. This would suggest that the rate of hydrogen abstraction for PINO is less than that of a peroxide radical. If this was not the case, the degree of degradation would be roughly equal because the macroradical they produce is virtually identical, meaning the rate of β-scission would be expected to be comparable for both. This is not reflected in the MFI data.
Nevertheless, there may be alternative mechanisms influencing the macro radical, thereby competing with β-scission. One such possible mechanism is epimerization. Since the carbon radical in the PP backbone changes hybridization from sp3 to sp2, the carbon in question adopts a flat, planar structure. As a result, another molecule or atom can bond on either side, with presumably equiprobable likelihood. There may be steric effects from the methyl groups though, since they are all pointing in the same direction in an isotactic configuration of PP of this investigation. If the molecule or atom bonds with the macro radical on the one side, it restores the stereo regularity; if it bonds on the other side, it introduces a stereo error in the chain.
Epimerization requires the stabilization of the macro radical before it can undergo β-scission. The only possibilities in the scheme to do this is chain transfer (Scheme 1, 5b) or (Scheme 1, 2c). That means that NHPI would have to be a chain transfer agent.
This would be reflected in the thermal properties, as this defect would reduce the crystallinity of the material. From DSC data (see supporting information) it is clear that the melt enthalpy reduces with increasing amounts of NHPI, although the differences are bordering on experimental error. Nevertheless, the DSC data suggests that epimerization plays indeed a relevant role in the mechanism. This is corroborated by NMR (Figure 5).
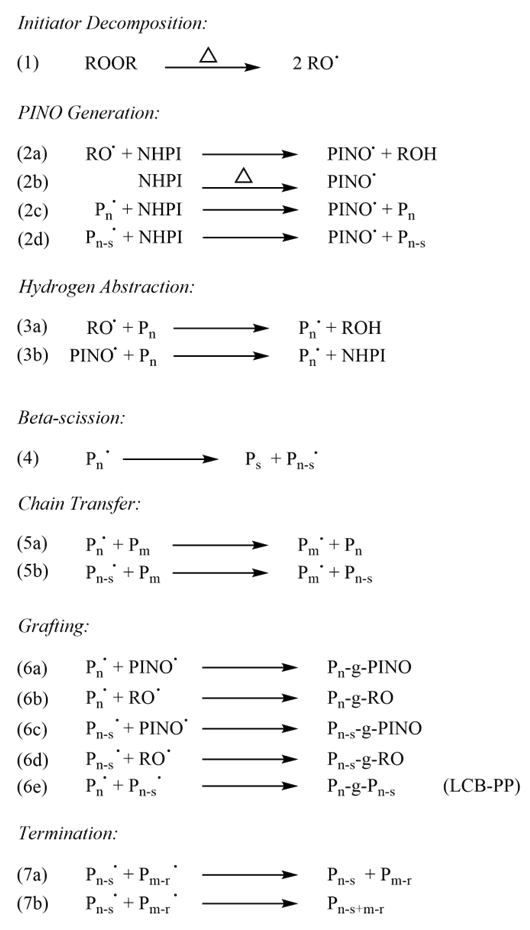
The isolated stereoerrors in Figure 5 are determined by quantifying the percentage of the mmmmrrmmm nonad. Since this percentage is going up with increasing amounts of NHPI, it can be said that NHPI has an effect to reduce the stereoregularity, by introducing stereoerrors. This proves the contribution of epimerization in the mechanism.
 Figure 5: NMR detected stereoerrors as function of NHPI content on a linear scale (left) and a logarithmic scale (right). All samples were treated with an active oxygen of 1000 PPM.
Figure 5: NMR detected stereoerrors as function of NHPI content on a linear scale (left) and a logarithmic scale (right). All samples were treated with an active oxygen of 1000 PPM.
However, as clearly as the data states that this happens, there has to be an explanation as to why this is the case. β-scission is fast, indeed most of the theoretical models of PP degradation assume it to be instantaneous whenever a hydrogen is abstracted (Berzin et al., 2000; Iedema et al., 2001; Tzoganakis et al., 1989). In this case however, the macroradicals seem to stay alive long enough for a hydrogen to have ample time to be returned. So, either the additional radicals have a stabilizing effect to reduce the kinetics of β-scission, or the increased concentration of these radicals promote the likelihood of competing reactions occurring due to a higher reaction rate.
It is most likely a kinetic effect due to an increased concentration. Considering the fast kinetics of β-scission, it is most likely to do with proximity; coagents need to be nearby in order for mass-transfer limitations to be mitigated as much as possible. NHPI melts at 233°C. At the operating conditions of this work of 200°C, NHPI is not molten. It seems unreasonable for PP to be such a good solvent that all the NHPI molecules dissolve throughout the polymer matrix. It is much more probable for there to be clusters of NHPI dispersed in the polymer matrix, even more so when you take into account the effects of π-π stacking of the aromatic rings. That means that multiple NHPI molecules are in very close proximity to each other. When one of the NHPI molecules is generated into PINO and abstracts a hydrogen from a PP chain, another NHPI is close by to donate its hydrogen and save the chain from β-scission. This is depicted in Scheme 1, where reaction (2c) is essentially reaction (3b) backwards.
This explanation might serve the observations made at N55/C55, but not what the NMR showed for N15, where the stereoregularity was also reduced, even though there is very little NHPI. As such, clusters of NHPI are not an explanation. However, the effects of β-scission on PP were studied, and one report found that the rate of change in MFI decreased with increasing amounts of peroxide (Tzoganakis et al., 1989). The report theorized that this was because increasing amounts of peroxide means increasing concentrations of radicals, which made it increasingly likely for one radical peroxide to abstract an H from the PP-backbone, and for a second to graft onto it. A similar trend can be observed in the present work, where initially the MFI is slowly plateauing, but not for a high concentration of peroxide; the rate of change seems to be increasing even beyond the range of the aforementioned report (Figure 2).
Upon consideration of the settings the authors used, it can be explained quite in line with what has been discussed so far. The authors used the same peroxide, but operated at 220°C and 20 RPM, whereas here the present work operates at 200°C and 30 RPM. At 220°C, the half-life time of the peroxide is lower than at 200°C, with the result that radicals will be generated at a faster rate, and so initially there will be more radicals at the same time compared to at 200°C with the same concentration of peroxide. Furthermore, at 20 RPM, the radicals are not dispersed in the PP melt as fast as at 30 RPM; due to a lower shear there is less mixing. The result is that their situation probably had more concentrated clusters of peroxide radicals compared to this work, making it more probable for one of the radicals to abstract an H and the other radical, the one it had just split from, to immediately graft onto the macroradical before β-scission can occur. Therefore, the MFI is kept below what it would have been if radicals were formed slower, or were dispersed quicker. As such, a similar plateau in MFI is not observed, because there would have been less concentrated clusters of peroxide radicals. However, at 1 wt% (~1000 PPM active oxygen, N15), a sufficient number of radicals will be present at the same time for peroxide grafting onto the PP backbone. This would affect the stereoregularity in exactly the same way as the NHPI cluster would. In this case, however, not through hydrogen donation, but via grafting of tert-butoxyl radicals.
The hypothesis stated at the end of the last section invokes a new mechanism: grafting of tert-butoxyl radicals. Nevertheless, there is no reason to presume grafting will be exclusive to this species; it may very well be any radical, which is why both PINO and a macroradical have also been included in Scheme 1. Note that it has been presumed that this is a secondary macroradical as product of β-scission, which seems more probably given the high rate of scission. This would result in long-chain branched PP (LCB-PP).
Reactions (7a) and (7b) would lead to the formation of C-O bonds, which would show up during FTIR. Reaction (7c) will affect the melt behavior, and so will show up during dynamic frequency sweep rheology.
However, the FTIR data for all samples is superimposable; therefore, there is no evidence of significant grafting of either PINO or the radical initiator. Both reactions (7a) and (7b) would result in a C=O bond, the band of which would show up around 1700 cm-1. This would also be a clear deviation from the starting material, and so it cannot be said that grafting has occurred, at least within the experimental detection limit of FT-IR (about 1%). This is not surprising as Opeida et al already noted this before (Opeida et al., 2019).
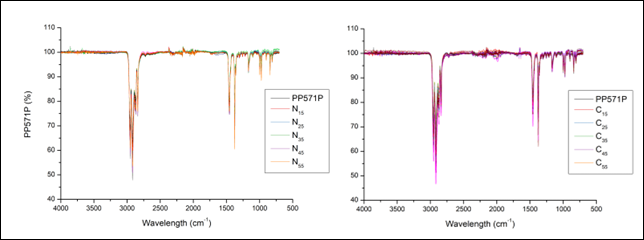 Figure 6: FTIR data
Figure 6: FTIR data
Branching wouldn’t necessarily introduce a significant chemical difference, since the branch is structurally the same as the backbone; therefore, FTIR is not going to give any insights about it. A technique that would is once again DFS rheology. The extrapolated zero-shear viscosities (Figure 4) are already discussed in terms of degradation. However, they may also give insights towards the effects of branching.
Since branching increases the MW of a single chain, the MWD shifts. Two polymer samples of similar MWD, where one is branched and the other is not, will show a higher zero-shear viscosity for the branched variant (Li et al., 2009; Maroufkhani & Golshan Ebrahimi, 2015). Since the MWDs of the samples are not the same due to effect of degradation, it is not conclusive to make statements about branching based on zero-shear viscosity alone. However, branched polymers also exhibit a stronger shear-thinning behaviour (Li et al., 2009; Maroufkhani & Golshan Ebrahimi, 2015).
Figure 7 depicts a representative sample compared to both the virgin PP and DaployTM, a commercially available LCB-PP. This figure shows that Daploy has a much steeper decrease in viscosity as the shear rate increases, which means that it is more shear-thinning. This behaviour is typical for LCB-PP (Li et al., 2009; Maroufkhani & Golshan Ebrahimi, 2015).
The virgin PP, PP571P, also shows shear-thinning, but much less compared to DaployTM. The representative sample, N22 shows a lower viscosity across the frequency sweep, indicating a reduced MW caused by β-scission, and shows a shear-thinning profile that is much more like the linear PP571P. All other samples exhibited similar behaviour; therefore, long chain branching can be ruled out.
 Figure 7: Frequency sweep of N22, virgin PP, and Daploy
Figure 7: Frequency sweep of N22, virgin PP, and Daploy
Under scCO2, the MFI is lower than without it, under the same conditions, whenever peroxide is used. In the cases where only NHPI is added it does not seem to make a difference; therefore, less degradation and less β-scission occurred because of the peroxide. Since β-scission is a unimolecular process, it probably wouldn’t be affected by the presence of scCO2, unless the radical would be stabilized by it. If that were the case, we’d also expect to see reactions (6) and (7a) in Scheme 1 to be more predominant, because they’ve got more time to happen and mass transfer of these agents to the macroradical is increased by scCO2. Another way to put it would be to state that the concentration of macroradicals would have to be higher if their lifetime is prolonged by scCO2. Both these reactions affect the stereoregularity, and this would be reflected in the thermal properties. Nevertheless, the DSC and NMR both point out that epimerization is less predominant when using scCO2, so these reactions are not as predominant. Therefore, it seems unlikely that scCO2 stabilizes the macroradical. Also, β-scission is the same for either NHPI or peroxide, these merely determine how the hydrogen is abstracted. If the macroradical were stabilized under scCO2, it would be expected to also see a reduction when using NHPI alone, which isn’t the case. And since the effect is seen across the board, it is much more likely that the scCO2 plays a role in one of the first steps, such as decomposition of the peroxide, or some solvent cage effects on the ensuing radical that reduces the rate of hydrogen abstraction.
NHPI is to some extend soluble in scCO2, so there is more NHPI available for the radical peroxide to react with. Since it seems likely that PINO isn’t as fast in abstracting a hydrogen as the peroxide radical, this would also explain why there is less degradation with than without scCO2. Better dispersion of NHPI would also make it even more likely for a peroxide radical to take a hydrogen from NHPI rather than PP, i.e., reaction (2a) is more prevalent compared to (3a) as the concentration is effectively higher, because NHPI molecules would be shielded by their peers inside a cluster.
Solubility would also explain why the stereoregularity hasn’t decreased as much using scCO2 compared to without at the same conditions. If scCO2 breaks up the cluster, there might not be a second NHPI molecule nearby to donate a hydrogen to the macroradical. Given this lack of competition, β-scission wins out by default.
Throughout this work, the mechanism (Scheme 1) has been elaborated upon bit by bit. Reactions (1), (3a), (4), (5a&b), and (7a&b) are well reported in literature about peroxide degradation of PP (Berzin et al., 2000; Iedema et al., 2001; Tzoganakis et al., 1989). In this work, NHPI has been added into the system and the following has been observed:
NHPI can increase MFI when there is little competition from another radical source; therefore, reaction (3b) was derived, where NHPI can degrade PP by H-abstraction as PINO (followed by reaction (4)). Additionally, reactions (2) have been postulated as possible ways to generate PINO from NHPI.
When competition is high, the presence of NHPI will decrease MFI. Given the propensity of PINO to degrade PP, this has been attributed to a probable kinetic effect: the peroxide radical reacts faster than PINO, i.e., reaction (3a) is faster than reaction (3b).
At high active oxygen, the stereoregularity is reduced as per DSC and NMR. Reaction (2c) explains some of this, where NHPI donates a hydrogen. The macroradical is planar (sp2 hybridized), therefore there is a roughly equal probability for epimerization, which would decrease the stereoregularity. Given the lack of evidence from the FTIR regarding grafting, reactions (6a&b), the drop in stereoregularity is more likely due to epimerization instead.
Dynamic frequency sweep rheology corroborated the trend MFI showed, whereas it did not find evidence to suggest long-chain branching.
Epimerization requires multiple NHPI molecules in close proximity, due to the fast nature of reaction (4) which directly competes with reaction (2b). Therefore, proximity is required for (2b) to be fast enough. The use of scCO2 can break up clusters of NHPI, which might explain a lower stereoregularity compared to the case where scCO2 is absent. On the other hand, reaction (3a) was also slower under scCO2, which might be due to solvent cage effects around the peroxide radical, blocking it temporarily from the polymer chain.
The authors would like to thank SNN and the Province of Groningen for funding through the European Fund for Regional Development platform. They furthermore are grateful for the insights offered by Klaas Remerie, Frits Zandvoort, Waldo Beek, Gert Boven, Rob Duchateau, and Daniele Parisi.
- Hara, T., Iwahama, T., Sakaguchi, S. & Ishii, Y. (2001). Catalytic oxyalkylation of alkenes with alkanes and molecular oxygen via a radical process using N-hydroxyphthalimide. Journal of Organic Chemistry, 66(19), 6425–6431. DOI: https://doi.org/10.1021/jo0157977
- Liang, Y. F., & Jiao, N. (2017). Oxygenation via C-H/C-C Bond Activation with Molecular Oxygen. Accounts of Chemical Research, 50(7), 1640–1653. DOI: https://doi.org/10.1021/acs.accounts.7b00108
- Lin, R., Chen, F. & Jiao, N. (2012). Metal-free, NHPI catalyzed oxidative cleavage of C-C double bond using molecular oxygen as oxidant. Organic Letters, 14(16), 4158–4161. DOI: https://doi.org/10.1021/ol3018215
- Matsunaka, K., Iwahama, T., Sakaguchi, S. & Ishii, Y. (1999). A Remarkable Effect of Quaternary Ammonium Bromide for the N-Hydroxyphthalimide-Catalyzed Aerobic Oxidation of Hydrocarbons. TETRAHEDRON LETTERS,40(11), 2165-2168. DOI: https://doi.org/10.1016/S0040-4039(99)00139-2
- Minisci, F., Recupero, F., Cecchetto, A., Gambarotti, C., Punta, C., Paganelli, R., Pedulli, G. F. & Fontana, F. (2004). Solvent and temperature effects in the free radical aerobic oxidation of alkyl and acyl aromatics catalysed by transition metal salts and N-hydroxyphthalimide: New processes for the synthesis of p-hydroxybenzoic acid, diphenols, and dienes for liquid crystals and cross-linked polymers. Organic Process Research and Development, 8(2), 163–168. DOI: https://doi.org/10.1021/op034137w
- Mo, Y. & Jensen, K. F. (2018). Continuous N-Hydroxyphthalimide (NHPI)-Mediated Electrochemical Aerobic Oxidation of Benzylic C−H Bonds. Chemistry – A European Journal, 24(40), 10260–10265. DOI: https://doi.org/10.1002/chem.201802588
- Opeida, I. A., Kompanets, M. A., Kushch, O. V, Papayanina, E. S. & Litvinenko, L. M. (2011). ACTION OF N-HYDROXYPHTHALIMIDE ON CHAIN STEREOREGULARITY IN THE RADICAL POLYMERIZATION OF METHYL METHACRYLATE. Theoretical and Experimental Chemistry, 47(1), 30-35.
DOI: http://dx.doi.org/10.1007/s11237-011-9180-3 - Opeida, I. O., Kushch, O. V., Kompanets, M. O., Litvinov, Y. E., Zosenko, O. O. & Shendrik, A. N. (2019). The Oxidative Polymerization of Vinyl Monomers in the Presence of N-Hydroxyphthalimide. ChemistrySelect, 4(40), 11826–11832. DOI: https://doi.org/10.1002/slct.201902597
- Koshino, N., Cai, Y. & Espenson, J. H. (2003). Kinetic study of the phthalimide N-oxyl (PINO) radical in acetic acid. Hydrogen abstraction from C-H bonds and evaluation of O-H bond dissociation energy of N-hydroxyphthalimide. Journal of Physical Chemistry A, 107(21), 4262–4267.
DOI: https://doi.org/10.1021/jp0276193 - Amaoka, Y., Kamijo, S., Hoshikawa, T. & Inoue, M. (2012). Radical amination of C(sp3)-H bonds using N -hydroxyphthalimide and dialkyl azodicarboxylate. Journal of Organic Chemistry, 77(22), 9959–9969. DOI: https://doi.org/10.1021/jo301840e
- Chen, L., Yue, F. S., Zhao, Y. M., Wang, S. S., Li, Y. C., Li, G. & Ge, X. C. (2021). Surface tailoring of polypropylene separators for lithium-ion batteries via N-hydroxyphthalimide catalysis. European Polymer Journal, 152. DOI: https://doi.org/10.1016/j.eurpolymj.2021.110487
- Zhou, H., Wang, S., Huang, H., Li, Z., Plummer, C. M., Wang, S., Sun, W. H. & Chen, Y. (2017). Direct Amination of Polyethylene by Metal-Free Reaction. Macromolecules, 50(9), 3510–3515. DOI: https://doi.org/10.1021/acs.macromol.6b02572
- Zhou, H., Plummer, C. M., Li, H., Huang, H., Ma, P., Li, L., Liu, L. & Chen, Y. (2019). Regioselective post-functionalization of isotactic polypropylene by amination in the presence of: N -hydroxyphthalimide. Polymer Chemistry, 10(5), 619–626. DOI: https://doi.org/10.1039/c8py01344f
- Culica, M. E., Kasperczyk, K., Baron, R. I., Biliuta, G., Macsim, A. M., Lazea-Stoyanova, A., Orlinska, B. & Coseri, S. (2019). Recyclable polymer-supported N-Hydroxyphthalimide catalysts for selective oxidation of pullulan. Materials, 12(21). DOI: https://doi.org/10.3390/ma12213585
- Gao, B., Meng, S. & Yang, X. (2015). Synchronously synthesizing and immobilizing N-hydroxyphthalimide on polymer microspheres and catalytic performance of solid catalyst in oxidation of ethylbenzene by molecular oxygen. Organic Process Research and Development, 19(10), 1374–1382. DOI: https://doi.org/10.1021/ACS.OPRD.5B00108/ASSET/IMAGES/MEDIUM/OP-2015-001089_0016.GIF
- Jian, M., Cui, J., Luo, D. & Xie, M. (2016). Catalytic properties of N-hydroxyphthalimide immobilized on a novel porous organic polymer in the oxidation of toluene by molecular oxygen. RSC Advances, 6(72), 68170–68177. DOI: https://doi.org/10.1039/C6RA14936G
- Lee, C., Dong, D., Beren, J. R., De Nicola, A. J. Jr., Mehta, S. D., Dang, V. A. & Fezza, R. J. (2013). Extrusion processes using high melt strength polypropylene (Patent US 8,546,504 B2). Retrieved from https://www.researchgate.net/publication/302671430_Extrusion_processes_using_high_melt_strength_polypropylene/fulltext/578cff3908ae59aa6681516f/302671430_Extrusion_processes_using_high_melt_strength_polypropylene.pdf?origin=publication_detail
- Scheve, J. B., Mayfield, J. W. & DeNicola, A. J. Jr. (1990). High melt strength, propylene polymer, process for making it, and use thereof (Patent US4916198A). Retrieved from https://patents.google.com/patent/US4916198A/en
- Scheve, J. B., Mayfield, J. W. & DeNicola, A. J. Jr. (1998). High melt strength, propylene polymer, process for making it, and use thereof (Patent US5731362A). Retrieved from https://patents.google.com/patent/US5731362A/en
- Coiai, S., Augier, S., Pinzino, C. & Passaglia, E. (2010). Control of degradation of polypropylene during its radical functionalisation with furan and thiophene derivatives. Polymer Degradation and Stability, 95(3), 298–305. DOI: https://doi.org/10.1016/j.polymdegradstab.2009.11.014
- Coiai, S., Passaglia, E. & Cicogna, F. (2019). Post-polymerization modification by nitroxide radical coupling. Polymer International, 68(1), 27–63.
DOI: https://doi.org/10.1002/pi.5664 - Passaglia, E., Coiai, S. & Augier, S. (2009). Control of macromolecular architecture during the reactive functionalization in the melt of olefin polymers. In Progress in Polymer Science (Oxford) 34(9), pp. 911–947. Pergamon. DOI: https://doi.org/10.1016/j.progpolymsci.2009.04.008
- Nalawade, S. P., Picchioni, F. & Janssen, L. P. B. M. (2006). Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Progress in Polymer Science (Oxford), 31(1), 19–43. DOI: https://doi.org/10.1016/j.progpolymsci.2005.08.002
- Picchioni, F. (2014). Supercritical carbon dioxide and polymers: An interplay of science and technology. Polymer International, 63(8), 1394–1399.
DOI: https://doi.org/10.1002/pi.4722 - Iedema, P. D., Remerie, K., van der Ham, M., Biemond, E. & Tacx, J. (2011). Controlled peroxide-induced degradation of polypropylene in a twin-screw extruder: Change of molecular weight distribution under conditions controlled by micromixing. Chemical Engineering Science, 66(22), 5474–5486. DOI: https://doi.org/10.1016/j.ces.2011.06.071
- Ryu, S. H., Gogos, C. G. & Xanthos, M. (1991a). Melting behaviour of controlled rheology polypropylene. Polymer, 32(13), 2449–2455.
DOI: https://doi.org/10.1016/0032-3861(91)90088-Z - Ryu, S. H., Gogos, C. G. & Xanthos, M. (1991b). Parameters affecting process efficiency of peroxide‐initiated controlled degradation of polypropylene. Advances in Polymer Technology, 11(2), 121–131. DOI: https://doi.org/10.1002/adv.1991.060110205
- Tzoganakis, C., Vlachopoulos, J. & Hamielec, A. E. (1988). Production of controlled‐rheology polypropylene resins by peroxide promoted degradation during extrusion. Polymer Engineering & Science, 28(3), 170–180. DOI: https://doi.org/10.1002/pen.760280308
- Tzoganakis, C., Hamielec, A. E., Tang, Y. & Vlachopoulos, J. (1989). Controlled degradation of polypropylene: A comprehensive experimental and theoretical investigation. Polymer-Plastics Technology and Engineering, 28(3), 319–350. DOI: https://doi.org/10.1080/03602558908048602
- Vergnes, B. & Berzin, F. (2000). Peroxide-controlled degradation of poly(propylene): Rheological behaviour and process modelling. Macromolecular Symposia, 158(1), 77–90. DOI: https://doi.org/10.1002/1521-3900(200008)158:1<77::AID-MASY77>3.0.CO;2-3
- Pfaendner, R. (2006). Nitroxyl radicals and nitroxylethers beyond stabilization: radical generators for efficient polymer modification. In Comptes Rendus Chimie 9(11–12), pp. 1338–1344. DOI: https://doi.org/10.1016/j.crci.2006.08.001
- Borsig, E., Lazár, M., Fiedlerová, A., Hrčková, L., Rätzsch, M. & Marcinčin, A. (2001). Solid-state polypropylene grafting as an effective chemical method of modification. Macromolecular Symposia, 176(1), 289–298. DOI: https://doi.org/10.1002/1521-3900(200112)176:1<289::AID-MASY289>3.0.CO;2-V
- Borsig, E., van Duin, M., Gotsis, A. D. & Picchioni, F. (2008). Long chain branching on linear polypropylene by solid state reactions. European Polymer Journal, 44(1), 200–212. DOI: https://doi.org/10.1016/j.eurpolymj.2007.10.008
- Liu, T., Hu, G. H., Tong, G. S., Zhao, L., Cao, G. P. & Yuan, W. K. (2005). Supercritical carbon dioxide assisted solid-state grafting process of maleic anhydride onto polypropylene. Industrial and Engineering Chemistry Research, 44(12), 4292–4299. DOI: https://doi.org/10.1021/ie0501428
- Rätzsch, M., Arnold, M., Borsig, E., Bucka, H. & Reichelt, N. (2002). Radical reactions on polypropylene in the solid state. In Progress in Polymer Science (Oxford) 27(7). https://doi.org/10.1016/S0079-6700(02)00006-0
- Nouryon. (n.d.). Initiators for Thermoplastics. Retrieved 4 July 2020, from https://www.nouryon.com/globalassets/inriver/resources/brochure-initiators-for-thermoplastics-en_us.pdf
- Seavey, K. C., Liu, Y. A., Khare, N. P., Bremner, T. & Chen, C. C. (2003). Quantifying relationships among the molecular weight distribution, non-Newtonian shear viscosity, and melt index for linear polymers. Industrial and Engineering Chemistry Research, 42(21), 5354–5362.
DOI: https://doi.org/10.1021/ie021003i - Wu, W., Jiang, W., Fu, Z., Xiao, J., Yuan, D. & Xing, C. (2023). Comparison of Dynamic and Steady Viscosity in High-Viscosity Modified Asphalt Binder and Analysis of Its Zero Shear Viscosity Test Methods. Journal of Materials in Civil Engineering, 36(2), 04023574.
DOI: https://doi.org/10.1061/JMCEE7.MTENG-16635 - Busico, V., Cipullo, R., Mingione, A. & Rongo, L. (2016). Accelerating the Research Approach to Ziegler-Natta Catalysts. Industrial and Engineering Chemistry Research, 55(10), 2686–2695. DOI: https://doi.org/10.1021/acs.iecr.6b00092
- Busico, V. & Cipullo, R. (2001). Microstructure of polypropylene. Progress in Polymer Science, 26(3), 443–533. DOI: https://doi.org/10.1016/S0079-6700(00)00046-0
- Gahleitner, M., Wolfschwenger, J., Bachner, C., Bernleitner, K. & Neißl, W. (1996). Crystallinity and Mechanical Properties of PP-Homopolymers as Influenced by Molecular Structure and Nucleation. Journal of Applied Polymer Science, 61(4), 649–657. DOI: http://dx.doi.org/10.1002/(SICI)1097-4628(19960725)61:4%3C649::AID-APP8%3E3.0.CO;2-L
- Berzin, F., Vergnes, B., Dufossé, P. & Delamare, L. (2000). Modeling of peroxide initiated controlled degradation of polypropylene in a twin screw extruder. Polymer Engineering and Science, 40(2), 344–356. DOI: https://doi.org/10.1002/pen.11168
- Iedema, P. D., Willems, C., Van Vliet, G., Bunge, W., Mutsers, S. M. P. & Hoefsloot, H. C. J. (2001). Using molecular weight distributions to determine the kinetics of peroxide-induced degradation of polypropylene. Chemical Engineering Science, 56(12), 3659–3669. DOI: https://doi.org/10.1016/S0009-2509(01)00054-9
- Coseri, S. (2009). Phthalimide-N-oxyl (PINO) radical, a powerful catalytic agent: Its generation and versatility towards various organic substrates. In Catalysis Reviews – Science and Engineering (Vol. 51, Issue 2, pp. 218–292). DOI: https://doi.org/10.1080/01614940902743841
- Li, S., Xiao, M., Wei, D., Xiao, H., Hu, F. & Zheng, A. (2009). The melt grafting preparation and rheological characterization of long chain branching polypropylene. Polymer, 50(25), 6121–6128. DOI: https://doi.org/10.1016/j.polymer.2009.10.006
- Maroufkhani, M. & Golshan Ebrahimi, N. (2015). Melt rheology of linear and long-chain branched polypropylene blends. Iranian Polymer Journal (English Edition), 24(9), 715–724. DOI: https://doi.org/10.1007/s13726-015-0357-9
- Su, F. H. & Huang, H. X. (2011). Supercritical carbon dioxide-assisted reactive extrusion for preparation long-chain branching polypropylene and its rheology. Journal of Supercritical Fluids, 56(1), 114–120. DOI: https://doi.org/10.1016/j.supflu.2010.12.001
- Wang, K., Wang, S., Wu, F., Pang, Y., Liu, W., Zhai, W. & Zheng, W. (n.d.). A new strategy for preparation of long-chain branched polypropylene via reactive extrusion with supercritical CO2 designed for an improved foaming approach. Journal of Materials Science, 51.
DOI: https://doi.org/10.1007/s10853-015-9584-x













