Article / Research Article
1Department of Anatomical Sciences, faculty of medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Hamideh Gharamaleki, Department of Anatomical Sciences, Faculty of medicine, Tabriz University of Medical Sciences
Tabriz, Iran
24 July 2020 ; 6 Aug 2020
Human beings are unavoidably exposed to ambient electromagnetic fields (EMF) generated from various electrical devices and from power transmission lines. Controversy exists about the effects of EMF on various organs. The aim of this work evaluation the effects of intrauterine exposure to 50Hz electromagnetic field (EMF) on testicular and ovary development.
Pregnant wistar rats were exposed to 3mT, EMF for 21 days, 4 hours/day. Pregnant rats under same condition of treatment group, but off the field as a sham group intended and pregnant rats were used as control in the room. After delivery, testis and ovary were removed from male and female pups, fixed and prepared for light microscopic studies.
Microscopic results revealed seminiferous tubules in treatment group in comparison with the control and sham groups were widely separated from each other, in this group in seminiferous tubules vacuolization, detachment of gonocytes from each other, heterochromatic gonocytes and decreasing in interstitial tissue was found. The ovary of the treatment group in comparison to control group showed that oocyte nests were mostly broken and irregularly arranged. The primordial follicles were less developed.
In general, as a result of the exposure to EMF during early developmental period, morphological changes in testicular and ovary development were evident, that may well extend till adult stage and may affect fertility.
Key words: EMF, Testis, Ovary, Development, Newborn
Development in technology have simplified human life, but exposure to Electromagnetic field (EMF) by using electrical machine, industrial instruments, communication devices, has occurred as a result of these technological development and has treats on our normal life [1]. EMF may be associated with detrimental effects on the human brain, cardiovascular system, and more specifically fetus organs pregnant women, and for these reasons these days the effects of EMF has gained more attention [2]. The possible effects of EMF on reproductive system have been studied both epidemiologically and experimentally. Experimental results suggest that EMF may disturb the development of chick embryos but the results are inconclusive [3].
Development of the gonad can be divided into two phases, an initial phase with formation of the bipotential gonad and a second phase with differentiation towards either testicular or ovarian fate [4]. Formation of functional gametes is essential for reproduction [5]. Primordial follicles, each consisting of an oocyte arrested in prophase me of meiosis and a single layer of flattened granulosa cells. This pool develops during fetal life in some species (e.g. primates, ruminants), but in others it develops during the early neonatal period (e.g. rodents, rabbits) [6]. Breakdown of germ-cell nests and formation of primordial follicles are key early events in mammalian folliculogenesis. Breakdown of germ-cell nests occurs during the same time window as the apoptotic death of approximately two-thirds of the oocytes within those nests; however, the mechanistic connection between these two events is not clear. It is believed that defects in the process of germ-cell nest breakdown leads to the formation of these multi-oocyte follicles [7]. In mice ovaries, programmed cyst breakdown occurs at 20.5–22.5 days’ post-coitus, and at the end of this period only 33% of oocytes can reach the stage of primordial follicle. The mechanism of germ cell death has been poorly understood, but has been viewed as a random process, which can be exacerbated by nutritional deficits or by environmental factors [8,9]. It has been reported that EMF exposure has a detrimental effect on the physiological parameters of the majority of exposed follicles and that this detrimental effect on the somatic (pre granulose) cells also [10,11].
Numerous studies have shown in mammals, the production of mature ova results from a coordinated sequence of events in the ovarian follicle involving various cell types, such as the oocyte and the surrounding granulosa theca cells. In newborn mouse, oocytes become enclosed in primordial follicle consisting of several enclosed granulosa cells surrounding each oocyte [12-14].
The seminiferous cords of the testis are composed of two distinct cell types; germ cells and a unique somatic supporting cell type, the sertoli cells [15]. The early phases of human development have scarcely been studied with regard to their correlation with EMF. Nevertheless, the very young should receive more attention because of greater fragility and susceptibility of the developing embryo, fetus and young child to environmental toxins of all kinds [16]. Studies show that when a pregnant woman is exposed to radiation, this radiation is also absorbed by the fetus, also studies show that though the fetus absorbs less than the mother, even for the fetus lower frequencies are absorbed more. This can expose the fetus to the same type of harmful effects that occur in adults [17]. Different studies show that radiation negatively impacts fertility in both males and females [18,19].
A number of studies showed that exposure to extremely low frequency EMF did not induce any adverse effects on spermatogenesis and reproductive capacity in experimental animals and human [20,21]. In contrast some studies conducted by other investigators showed clear damage to spermatogenesis [22-24]. Therefore, more careful and detailed studies need to be carried out to determine whether EMF exposure can induce adverse effects on spermatogenesis and reproductive capacity. Many studies have been carried out or are in progress about the various effects of radiation emissions regarding the behaviors, cancer central nervous system, sleep, children, cardiovascular system, immune function and development [25]. It did not find dominant lethal mutation in the male germ cells in mice when they were exposed to power frequency magnetic fields at 10mT for the approximate period of spermatogenesis [26]. It suggests that the effect of the exposure may delay gonocyte development, and decrease fetal body weight and testis weight [27].
Reproductive effects of low energy of EMF are less well defined, and mechanisms responsible for effects on reproduction and development are not well understood. 28 It is important to confirm animal research in humans so that future generations can be protected from the adverse effects of radiation exposure. In this study, it was aimed to investigate whether there were any toxic effects of ELF- EMF on the ovary and testis of rat pups?
In the present research, 18 female Wistar rats weighting 200-250g with 2-3 month age were studied. The rats were supplied from animal house of the histology Department Faculty of Medicine, Tabriz University of Medical Sciences. The rats were housed in plastic cages and kept under 12h light/dark condition under 20-220C, 50-60% humidity and free access to food and water. The rats were mated and pregnancy was determined by detection of vaginal plug. The pregnant rats were divided into 3 groups of 6 rats in each group, including: Treatment, Sham and Control.
The rat in treatment group were exposed to 3mT EMF produced by 50Hz and the rats were exposed for 4 hours/day during the pregnancy period. The sham group was kept in a similar condition without exposure to EMF. The control group was kept in standard condition. After delivery, female and male newborn pups were sacrificed and their gonads were dissected and prepared for microscopic studies.
The equipment was based on the Helmholtz coil, which operated following Fleming’s right hand rule. The equipment produced an alternating current of 50 Hz. The intensity of the EMF was controlled using a transformer. The equipment had two main parts. In the first part, there were two copper coils placed one above the other and separated by a distance of 50 cm. A cylindrical wooden vessel was placed between the coils (the exposure area), the interior contained a chamber for holding the caged experimental animals. The second part was the transformer, controlling the input and output voltage using a voltmeter and the current voltage with an ampere meter. A fan was used to prevent from increasing temperature inside the chamber. Four cages at a time were placed within the chamber, with 10 rats per cage.
Ovary and testis was fixed in10% formalin (Merck, Germany) for 48 hrs and processed for light microscopic studies. The samples were embedded in paraffin, and 5-µm thick sections were prepared and stained with hematoxylin and Eosin (H&E).
Light Microscopic study showed that in 1 day old neonatal rat ovary from the control group, oocytes and oocyte nests were already appeared clearly. The primary follicles have a large oocyte and a layer of granulosa cells [Figure 1].
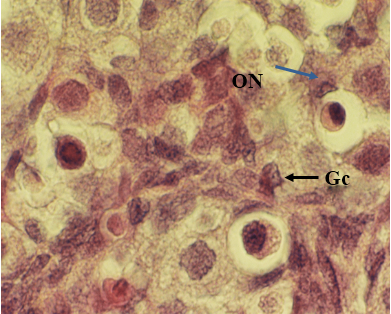 Figure 1: Light microscopic micrograph from ovary of 1 day old neonatal rat control group. Note differentiating oocyte nest (ON), primordial follicle and layer of Granulosa cells (Gc).1280 X, H & E
Figure 1: Light microscopic micrograph from ovary of 1 day old neonatal rat control group. Note differentiating oocyte nest (ON), primordial follicle and layer of Granulosa cells (Gc).1280 X, H & E
In the experimental group, numerous oocyte nests were in the stroma of the ovaries, the oocyte and the oocyte nest appeared to be fewer in comparison to that of the control group. Some oocytes were present as dispersed among stromal cells and few oocyte nests were broken and some primordial follicles were binucleated [Figure 2, 3].
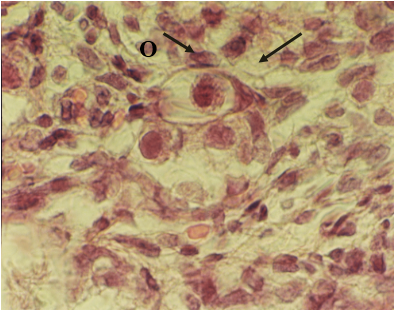 Figure 2: Light microscopic micrograph from neonatal female mouse exposed to EMF. Note scattered oocyte (O) and rapture in arrangement of pregranulosa cell (arrow) (H and E, ×1280X)
Figure 2: Light microscopic micrograph from neonatal female mouse exposed to EMF. Note scattered oocyte (O) and rapture in arrangement of pregranulosa cell (arrow) (H and E, ×1280X)
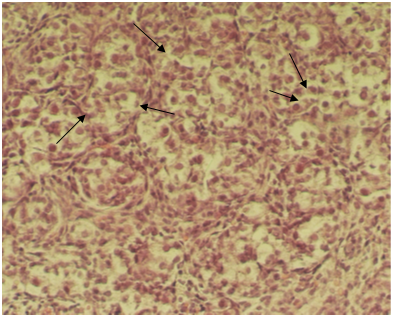 Figure 3: Light microscopic micrograph from neonatal female mouse exposed to EMF. Note oocytes nests with several oocyte(arrow), binucleated oocytes (double arrow) (H and E, ×256X)
Figure 3: Light microscopic micrograph from neonatal female mouse exposed to EMF. Note oocytes nests with several oocyte(arrow), binucleated oocytes (double arrow) (H and E, ×256X)
No pathological changes were observed in sham and control groups. Testicular sections from control rats revealed seminiferous cords comprised of sertoli cells that were mainly located at the central of the cords and primary germ cells (gonocytes) were located in the periphery of the cords. The cords were ere separated by connective tissue and enclosed by peritubula rmyoid cells (PMC) Figure 4.
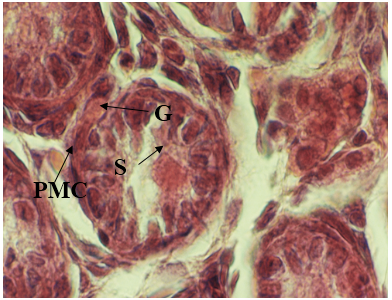 Figure 4: Light micrographs of seminiferous tubules of male, newborn rat in control group. Sertoli cell (S) and Gonocyte (G) was found. Normal interstitial tissue around seminiferous cords with peritubularmyoid cells (PMC) 1280 X, H&E
Figure 4: Light micrographs of seminiferous tubules of male, newborn rat in control group. Sertoli cell (S) and Gonocyte (G) was found. Normal interstitial tissue around seminiferous cords with peritubularmyoid cells (PMC) 1280 X, H&E
The results showed in EMF-exposed rats seminiferous cords were widely separated from each other. In this group vacuolization in seminiferous cords, detachment of gonocytes from each other, heterochromatic gonocytes and normal sertoli cells was found. Also vacuolization was found in interstitial tissue [Figure 5,6].
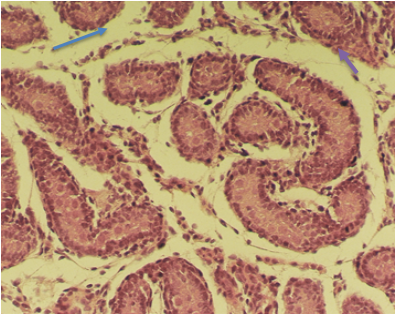 Figure 5: Light microscopic micrograph of seminiferous cords from newborn rats in treatment group (EMF-expose). Note seminiferous cords were widely separated from each other (arrow). 256 X, H& E
Figure 5: Light microscopic micrograph of seminiferous cords from newborn rats in treatment group (EMF-expose). Note seminiferous cords were widely separated from each other (arrow). 256 X, H& E
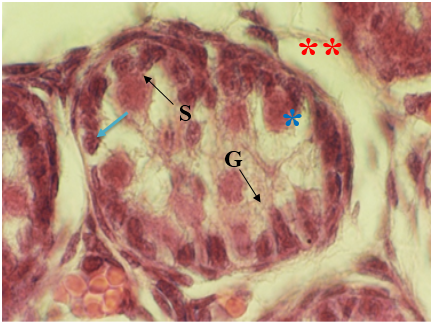 Figure 6: Light microscopic micrograph of seminiferous cords from newborn rats in treatment group (EMF-expose). Note vacuolization in seminiferous cords (*), detachment of gonocytes from each other(arrow), heterochromatic gonocytes (G), normal sertoli cells (S) and vacuolization in interstitial tissue (**). 1280 X, H & E
Figure 6: Light microscopic micrograph of seminiferous cords from newborn rats in treatment group (EMF-expose). Note vacuolization in seminiferous cords (*), detachment of gonocytes from each other(arrow), heterochromatic gonocytes (G), normal sertoli cells (S) and vacuolization in interstitial tissue (**). 1280 X, H & E
During the last decade, association has been suggested between chronic or long-term exposure to EMF and its toxic effects on reproduction [29,30]. Under the light of the previous results whether the exposure to EMF has any effects on reproduction and development has not exactly been defined yet [31,32]. Exposure to 6GHz RF radiation, there was developmental retardation in pups. Other studies using mice and rats have attempted to elucidate the reproductive toxic effects of exposure to low frequency magnetic field, and the results have been found to be inconsistent [33]. The interpretation of conflicting results on damage caused by low-frequency EMF on testis is not easy with the presence and application of different methods by different researchers, lack of adequate environment control during the experiment such as temperature, humidity etc. have reported adverse effects caused by exposure to electromagnetic field such as cytotoxic effects on spermatogonia, increased apoptosis, reduction in the count of sperm, fertility in rat and adverse effects on sperm quality in humans [34].
The degenerative changes in seminiferous tubules are exposure to ELF-EMF resulted in reduction in the number of well-organized seminiferous tubules, increased germ cell death and apoptosis [35]. Magnetic field exerts its action on the testis by various ways as change in enzymatic activity (as lactate dehydrogenase isoenzyme) within the testis, decrease in fatty acid composition of membrane phospholipids fraction of testicular cells, decrease DNA synthesis or gene expression changes which provoke disturbances in cell metabolism and DNA breaks [36].
Chung et al showed that exposure to EMF (from 60 Hz up to 500mT), both prenatally and postnatal, did not alter offspring spermatogenesis in the rat [37]. Electric and magnetic fields may cause toxic effects in various cell types, including germ cells [23,38]. EMF can influence the mechanism of apoptosis and also lead to increased apoptosis rate of germ cells [39,40]. Usually spermatogonia differentiate irreversibly; the only option for germ cells that will not become sperm is degeneration [41]. The present study also observed germ cells with morphological changes similar to apoptosis in animals exposed to EMF. In this study, presence of cells with dense and heterochromatin nuclei and their separation from neighboring cells were evident (Fig. 2, 3). In a similar our study, Hong et al, also concluded that 50Hz EMFs (0.2mT or 6.4mT, exposed for a period of 4 weeks) may have the potential to induce DNA strand breakage in testicular cells in mice [42]. These features are considered as pre-apoptotic signs and indicate that apoptosis induction occurs at exposure time [43].
This study questions whether electromagnetic radiation has any toxic effects on reproduction by causing changes in ovary of pups? We assume that the EMF can cause a decrease in the number of ovarian follicles in rat pups exposed to EMF during their intrauterine life. In utero exposure to cell phone radiation in rats reduces number of ovarian follicles in female offspring. In addition, there is evidence of miscarriages in female physical therapists exposed to this radiation [44].
The results obtained in the present study were binuclation of primordial follicles with irregular nuclei and broken oocyte. Breakdown of oocyte nests has already been described by [45,46]. The current study shows that EMF exposure increases degenerative changes and oocyte nest breakdown and follicular formation, undergo a series of incomplete cell division, resulting in clusters called cysts or nest [47].
In accordance with our findings Cecconi et al, has reported that, most of the nest cells differentiate as nurse cells and contribute to cytoplasm components, including ribosomes to the future oocyte [48,49]. It has been reported that EMF exposure has a detrimental effect on the physiological parameters of the majority of exposed follicles and that this detrimental effect on the somatic (pre granulose) cells also [50].
The results of this study indicated that exposure to EMF in prenatal period had a pathological change on the ovary and testis in neonatal. Therefore, it may lead to male subfertility and infertility in adult age. We suggest that further studies are required to be done on this subject.
- Bahaodini A, Owifard M, Tamadon A, Jafari SM (2015) Low frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels on male rats. Asian Pacific Journal of reproduction 4(3): 195-200.
- Alaa J Hamada, Singh A and Agarwal A (2011) Cell Phones and their Impact on Male Fertility: Fact or Fiction. The Open Reproductive Science Journal 5: 125-137.
- Huuskonen H, Juutilainen J, Julkunen A, Komulainen H (1998) Effects of Low-Frequency Magnetic Fields on Fetal Development in CBA/Ca Mice. Bioelectromagnetics 19: 477-485.
- Lisboa Gomes N, Chetty T, Jorgensen A, Mitchell R A (2020) Disorders of Sex Development—Novel Regulators, Impacts on Fertility, and Options for Fertility Preservation. International journal of molecular science 21(2282): 1-31.
- Chen Y, Breen K and Pepling EM (2009) Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. Journal of Endocrinology 202: 407–417.
- Fortune JE (1994) Ovarian Follicular Growth and Development in Mammals. Biology of reproduction 50: 225-232.
- Jingxia Xu, Thomas G (2013) Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. Biomed Central Biology 11(13): 1-13.
- Dean J (2002) Oocyte-speci Wc genes regulate follicle formation, fertility and early mouse development. J Reprod Immunol 53: 171–180.
- Epifano O, Dean J (2002) Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab 13:1 69–173.
- Meredith S, Dudenhoeffer G, Jackson K (2000) Classification of small type B/C follicles as primordial follicles in mature rats. J Reprod Fertil 119: 43 48.
- Torregrossa MV (2005) Biological and health effects on electric and magnetic fields at extremely low frequencies. Ann Ig 17: 441 453.
- Juutilainen J (2005) Developmental effects of electromagnetic fields. Bioelectromagnetics 7: 107 115.
- Pepling ME (2006) From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 44: 622 632.
- Roshangar L, Soleimani Rad J, Afsordeh K (2010) Maternal tamoxifen treatment alters oocyte differentiation in the neonatal mice: Inhibition of oocyte development and decreased folliculogenesis. J Obstet Gynaecol Res 36: 224 231.
- Griswold MD, C Morales, SR Sylvester (1998) Molecular biology of the Steroil cell. Oxford Reviews of Reproductive Biology, New York: Oxford University Press: 10: 120-161.
- Davis GE, Lowell WE (2008) Peaks of solar cycles affect the gender ratio. Med Hypotheses 71(6): 829-838.
- Nagaoka T, Togashi T, Saito K, Takahashi M (2007) An anatomically realistic whole-body pregnant-woman model and specific absorption rates for pregnant-woman exposure to electromagnetic plane waves from 10 MHz to 2 GHz. Physics in Medicine and Biology 52 (22): 6731-6745.
- Ash P (1980) The influence of radiation on fertility in man. British Journal of Radiology 53: 271-278.
- Gul A, Celebi H, and Ugras S (2009) The effects of microwave emitted by cellular phones on ovarian follicles in rats. Archives of Gynecology and Obstetrics 729-733.
- Ryan BM, Symanski RR, Pomeranz LE, Johnson TR, Gauger JR, McCormick DL (1999) Multigeneration reproductive toxicity assessment of 60 Hz magnetic fields using a continuous breeding protocol in rats. Teratology 59: 156 162.
- Heredia Rojas JA, Caballero Hernandez DE, Rodriguez de la Fuente AO, Ramos Alfano G, Rodriguez Flores LE (2004) Lack of alterations on meiotic chromosomes and morphological characteristics of male germ cells in mice exposed to a 60 Hz and 2.0 mT magnetic field. Bioelectromagnetics 25: 63 68.
- UNEP/WHO/IRPA (1987) United Nations Environment Programme/International Radiation Protection Association/World health Organization. Environmental health criteria 69: Magnetic fields. Geneva. WHO.
- De Vita R, Cavallo D, Raganella L, Eleuteri P, Grollino MG, Calugi A (1995) Effects of 50 Hz magnetic fields on mouse spermatogenesis monitored by flow cytometry analysis. Bioelectromagnetics 16: 330 334.
- Lee JS, Ahn SS, Jung KC, Kim YW, Lee SK (2004) Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl 6: 29 34.
- Valberg PA, Kavet R, Rafferty CN (1997) Can low level 50/60 Hz electric and magnetic fields cause biological effects? Radiat Res 148: 2 21.
- Kowalczuk CL, L Robbing, JM Thomes, RD Saunders (1995) Dominant lethal studies in mice after exposures to 50 Hz magnetic Field. Mutat Res 328: 229-237.
- Carnes KI, JL Drewniak, F Dunn (1991) In utero measurement of ultrasonically induced fetal mouse temperature increases. Ultrasound Med. Bio.
- Negishi T, Imai S, Itabashi M, Nishimura L, Sasano T (2002) Studies of 50 Hz circularly polarized magnetic Welds of up to 350 -T on reproduction and embryo–fetal development in rats: exposure during organogenesis or during implantation. Bioelectromagnetics 23: 369–389.
- Infante-Rivard C (1999) Electromagnetic Weld exposure during pregnancy and childhood leukemia. Lancet 346: 177–182.
- Adey WR (1993) Biological eVects of electromagnetic Welds. J Cell Biochemistry 51: 410–416.
- Svendnstal BM, Johnson KJ (1995) Fetal loss in mice exposed to magnetic Welds during early pregnancy. Bioelectromagnetics.
- Berman E (1990) The developmental effects of pulsed magnetic Welds on animal embryos. Report Toxically 4: 45–49.
- Juutilainen J (1991) Effects of low frequency electromagnetic fields on embryonic development and pregnancy. Scand J Work Environ Health 17: 149–158.
- Axdin M, G Turk, M Yuksel, A Gevilk, Apaydin A.M, Xilmazs (2007) Effect of electonomagnatic field on the sperm characteristics and histopathological status of testis in rats. Medxc.Weteryn 63: 178-183.
- Sert C, Akdag MZ, Bashan M, Buyukbayram H, and Dasdag S (2002) ELF magnetic field effects on fatty acid composition of phospholipid fraction and reproduction of rat’s testes. Electromagnetic Biology and Medicine 21: 19-29.
- Aisha A, Saad El-Din, Nabila A, Abd El-Motaal, Haidy F, Abd El Hamid, Yasser F. El-Akid. 2006. The Egyptian Journal of Hospital Medicine 24: 460 –476.
- Chung MK, Lee SJ, Kim YB, Park SC, Shin DH, Kim SH, Kim JC (2005) Evaluation of spermatogenesis and fertility in F1 male rats after in utero and neonatal exposure to extremely low frequency electromagnetic fields. Asian J Androl 7: 189–194.
- Ivancsits S, Pilger A, Diem E, Jahn O, Rüdiger HW (2005) Cell type‐specific genotoxic effects of intermittent extremely low‐frequency electromagnetic fields. Mutat Res 583: 184–188.
- Lai H, Singh NP (2004) Magnetic‐field‐induced DNA strand breaks in brain cells of the rat. Environ. Health Persp 112: 687–694.
- Kim YW, Kim HS, Lee JS, Kim YJ, Lee SK, Seo JN, Jung K‐C, Kim N, Gimm YM (2009) Effects of 60Hz 14 mT magnetic field on the apoptosis of testicular germ cell in mice. Bioelectromagnetics 30: 66–72.
- Russel LD, Ettlin RA, Sinha Hikim AP, Clegg ED (1990) Mammalian spermatogenesis. In Histological and Histopathological Evaluation of the Testis. Cache River Press: Bolesta 1–40.
- Hong R, Zhang V, Liu Y, Weng EQ (2005) Effects of extremely low frequency electromagnetic fields on DNA of testicular cells and sperm chromatin structure in mice 23(6): 414-417.
- Tablado L, Soler C, Nunez M, Nunez J, Perez-Sanchez F (2000) Development of mouse testis and epididymis following intrauterine exposure to a static magnetic field. Bioelectromagnetics 21: 19-24.
- Elbetieha A, Al-Akhras MA, Darmani H (2002) Long-term exposure of male and female mice to 50 Hz magnetic Weld: Effects onfertility. Bioelectromagnetics 23: 168–172.
- Pepling ME (2006) From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 44: 622 632.
- Roshangar L, Soleimani Rad J, Afsordeh K (2010) Maternal tamoxifen treatment alters oocyte differentiation in the neonatal mice: Inhibition of oocyte development and decreased folliculogenesis. J Obstet Gynaecol Res 36: 224 231.
- Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234: 339 351.
- Cecconi S, Gualtieri G, Di Bartolomeo A, Troiani G, Cifone MG, Canipari R (2000) Evaluation of the effects of extremely low frequency electromagnetic fields on mammalian follicle development. Hum Reprod 15: 2319 2325.
- Pourlis AF (2009) Reproductive and developmental effects of EMF in vertebrate animal models. Pathophysiology 16: 179 189.
- Meredith S, Dudenhoeffer G, Jackson K (2000) Classification of small type B/C follicles as primordial follicles in mature rats. J Reprod Fertil 119: 43 48.













