Article / Research Article
Department of Microbiology, Faculty of Science, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
Dr. Oludare Temitope Osuntokun
Department of Microbiology
Faculty of Science
Adekunle Ajasin University
Akungba-Akoko
Ondo State
Nigeria
1 July 2020 ; 20 July 2020
SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have animal based origin from Bats(Betacoronavirus) in subgenus (Sarbecoronavirus) together with two bat-derived strains The virus is mainly spread between people during physical contact, often through small droplets produced during coughing, sneezing, even talking to patient or infected people. It is primarily spread during close contact and via small droplets produced when infected people cough, sneeze or talk with close contact being within 1–3 m (3 ft 3 in–9 ft 10 in). Those infected with the virus may be asymptomatic (Appearance of No symptoms) or develop flu-like symptom which include fever, cough, fatigue, and shortness of breath. The exposure time to initial symptom is day five (5 days). Majority of cases result in mild symptoms, some progress to viral pneumonia and multiple organ failure. Two medicinal plants may be used for the treatment of SARS-CoV-2, the two medicinal plants are Aframomum melegueta and Spondis mombin (Linn). Both contains arrays of natural product that may be used for drug formulation to combat SARS-CoV-2, Aframomum melegueta medicinal plant belong to the family Zingiberaceae which can be used to detoxify liver and bronchitis during lungs infection. Aframomum melegueta contains 6-Gingerol, 8-Gingerol, Methyl-6-Gingerol, 6-Shogaol, 6-Rac-6-Dihydro paradol,6-Gingeredione, 2-(5 butylfuran-2-yl) ethyl}-2-methoxyphenol and 6-Paradol.
Secondary metabolite(Phytochemical)investigations of Aframomum melegueta extracts revealed the presence of (-)-buplerol, (-)-arctigenin, (E)- 14-hydroxy-15-norlabda-8(17), 12-dien- 16-al, labda-8(17), 12-dien-15, 16- dial, 16-oxo-8(17), 12(E)-labdadien-15-oic acid, 5-hydroxy-7-methoxy flavone and apigenin, The combination of all this novel compound can be used for the treatment of SARS-CoV-2. Spondias mombin belongs to the family of Anacardiaceae medicinal plants and contains Catechin and Epigalocatechin, Epicatechin, Stigmasterol and Phytosterol, a very important flavonoid that can be used in drug formulation because of its extensive therapeutic properties which may inhibit specific enzymes, blocking the angiotensin-converting enzyme (ACE) that raises blood pressure of infected patient during elevated fever clinical symptoms of SARS-CoV-2 (COVID-19). Spondias monbin extract may act by blocking the “suicide” enzyme cyclooxygenase that breaks down prostaglandins, they also prevent platelet aggregation. Cancer-battling antioxidants, flavonoids, have been found in relative abundance in Aframomum melegueta and Spondias mombin. Epicatechin have been proven to have diverse benefits to human health. It reduces the risks of diabetes mellitus and cardiovascular diseases, EGC acts as a strong inhibitor of HIV replication in cultured peripheral blood cells and inhibition of HIV-1 reverse transcriptase in vitro. EGC binds directly to CD4 molecules with consequent inhibition of Gp 120 binding and inactivate viruses in vitro by deformation of phospholipids, Epicatechin and other polyphenols decreases the susceptibility of low density lipoprotein to oxidation which prevents the initiation of artherosclerosis, HIV protein (Tat) and gp120.In conclusion. SARS-CoV-2(COVID-19) pandemic infection should not be a death sentence, it should be an advancement into the scientific world especially the naturally occurring product that can be used in the formulation of new antimicrobial agents.
Keywords: Aframomum Melegueta, Spondias mombin, SARS-CoV-2 (COVID-19)
SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have animal based origin from Bats, Betacoronavirus and Sarbecoronavirus together with two bat-derived strains [27,28,29]. Coronavus disease 2019 (COVID-19) is a very infectious symptomatic infection caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SAR-COV-2) [1]. The infection sequentially was first identified in December 2019 in the chaina capital called Wuhan [2]. The Common symptoms include high fever, Cough, and shortness of breath, just to mention a few. Other symptoms may include fatigue, muscular pain, diarrhea, sore throat, loss of smell, and abdominal discomfort/pain [3]. The exposure time to initial symptom is day five (5 days), this may range from two to fourteen days as the infection sequentially develop to diseases [4,5]. Majority of cases result in mild symptoms, some progress to viral pneumonia and multiple organ failure, but not in all case all case eventually leads to death of the patient within a short time [6].
The virus is mainly spread between people during close contact (Horizontal transfer) often via droplets produced during coughing, sneezing, or talking [7,8]. While these droplets are produced when breathing out in to the air, they usually fall to the surfaces rather than being infection over large distance [9]. People may also become infected by touching a contaminated surface I,e inanimate object and then their face and nose or the orifice in the body [10]. The virus can survive on surfaces for up to 72 hours within going to the death phase [11,12].
Those infected with the SARS-CoV-2 virus may be asymptomatic (Appearance of No symptom) and develop flu-like symptom during the progreasion of infection. Other symptoms include high fever, continuous cough, fatigue (Tiredness), and shortness of breath due to congestion of lung cavity, persistent chest pain or pressure, confusion, difficulty waking [17,18]. In some cases, other less commonly symptoms like upper respiratory pain, such as sneezing, runny nose or sore throat. Other such as nausea, vomiting and diarrhea have been observed in varying percentages of infected patient [19].
The incubation period for SARS-CoV-2 (COVID-19) is typically five to six days (5-6 days) but may range from two to 14 days in all, as symptoms progress in subclinical phase II infection [20]. Preliminary evidence suggests asymptomatic cases may contribute to the spread of SARS-CoV-2 (COVID-19), it is primarily spread during close contact and by small droplets produced when people cough, sneeze or talking with close contact (Physical/social contact) being within 1–3 m (3 ft 3 in–9 ft 10 in) [21].
However, SARS-CoV-2 (COVID-19) pandemic infection varies based on the humidity and temperature of the infected country. High humid/ high temperate country as less susceptible to SARS-CoV-2 (COVID-19) compared with less humid/temperate country. To reduce rampaging effect of SARS-CoV-2 (COVID-19) pandemic infection, Surfaces may be decontaminated with a number of antimicrobial solutions within one minute of exposure to the disinfectant to achieve a sterile inenvironment free of the SARS-CoV-2 (COVID-19) virus. Some of the antimicrobial agent are ethanol, 2-propanol, sodium hypochlorite (bleach), hydrogen peroxide, povidone– iodine, Soap and detergent. 78–95% ethanol (alcohol used in spirits), 70–100% 2-propanol (isopropyl alcohol), the combination of 45% 2-propanol with 30% 1-propanol, 0.21% sodium hypochlorite(bleach), 0.5% hydrogen peroxide or 0.23–7.5% povidone–iodine. Soap and detergent are also effective if correctly used. Soap products can be used to degrade and deactivating the virus fatty protective layers, as well as freeing them from skin and other surfaces [23,24,25].
The standard method of diagnosis the SARS-CoV-2 (COVID-19) pandemic infection is by Real time reverse transcription Polymerase Chain Reaction (Rrt-PCR), by taking a nasopharyngeal swab [13]. Another method of diagnose SARS-CoV-2 is by making use of Chest CT imaging from suspected individual based on symptoms and risk factors but is not recommended for routine screening [14,22].
The lungs are the organs most affected by SARS-CoV-2 (COVID-19) because the virus accesses host cells via the enzyme anglotensin–converting enzymes 2 (ACE2), which is most abundant in the type I and II alveola cells of the lungs. The virus uses a special surface glycoprotein called a “spike” (peplomer) to connect to ACE2 and enter the host cell [29]. The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested that decreasing ACE2 activity might be protective, though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective and that these hypotheses need to be tested [30]. As the alveolar disease progresses, respiratory failure might develop and death may follow [31]. The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium as well as endotheli cells and Enterocytes of the small intestine [32].
SARS-COV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, infected patients with SARSCoV-2 (COVID-19) have symptoms of systemic hyperinflammation. Clinical laboratory findings shows varing degree of elevated IL-2, IL-7, IL-6, granulocyte macrophages stimulating factors (GM-CSF) and this include the interferonsgamma, inducible protein 10(IP-10),macrophage inflammatory protein (Alpha 1(MIP-1Alpha) and tumor necrosis factor alpha that can inductive of cytokine released syndrome [33]. It should be noted that if all this factors can be blocked by a medicinal plants, then, we can say we have a cure and treatment for this world pandemic, this is the basic reason this review becomes very important to the scientific world.
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung of the infected patient, it should be noted that the lung and heart of infected patient are mostly affected. In particular, pathogenic GM-CSF-secreting T-cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in SARS-CoV-2 (COVID-19) infected patients [34]. It there is any medicinal plant that can lower the IL-2, IL-7, IL-6 and pathogenic GM-CSF-secreting T-cells, then we have a breakthrough on SARS-COV-2 infection prevention is better than cure, so say an adage,Preventive measures can be established to reduce the scourge of SARSCOV-2, this preventive measures include staying at home, which is mostly recommended in all cases, to prevent the spread of infection and avoiding crowded places, like church gathering, school market places, train station, airport. Washing of hands with soap and water regularly, for at least 20 seconds, practicing good respiratory hygiene and avoiding touching the eyes, nose or mouth and other orifice in the body with unwashed hands [35,36]. People are managed with supportive care which may include fluid therapy, oxygen support and ventilator [37,38]. Those people suspected they carry the virus wear a simple face mask that will cover their mouth and nose. The ability to spread SARS-COV-2(COVID-19) varies from individual to individual, and country to country. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold [15,16,39].
Several existing antiviral medications are being evaluated for treatment of COVID-19, including clhloroquine and hydrooxy-chloroquine [40]. Others are lopinavi, ritonavir with combination of Beta interferon (41). Remdesivir inhibits SARS-CoV-2 in vitro evidence of clinical trial conducted in the United States, Italy and China [42-44]. Chloroquine, previously used to treat malaria fever [43]. Chloroquine phosphate “improves the success rate of treatment and shortens the length of person’s hospital stay” and recommended it for people diagnosed with mild, moderate and severe cases of novel coronavirus pneumonia [45]. There are mixed results as to the effectiveness of hydroxychloroquine as a treatment for COVID-19, with some studies showing little or no improvement, hence there is a need of alternative source of treatment this is where medicinal plants come into vintage position.
Medicinal plants has become an obsolete version of the synthetic drug, but come to think of it its uses a limitless, that is the basic reason it will become a veritable tools in the treatment of recalcitrant infection like SARS-CoV-2 (COVID-19). This section of this review will shed more light to just three (3) medicinal plants found in Nigeria which may be used to treat and cure SARS-CoV-2 (COVID-19) and its allies if the foreign world takes cognizant of its uses and various application.
Possible medicinal plants that may be used for the treatment of SARS-CoV-2 (COVID-19).
From literature, Aframomum melegueta medicinal plant belong to the family Zingiberaceae which can be used to detoxify liver and bronchitis during lungs infection. Aframomum melegueta contains 6-Gingerol, 8-Gingerol, Methyl-6-Gingerol, 6-Shogaol,6-Rac-6-Dihydro paradol,6-Gingeredione, 2-(5 butylfuran-2-yl) ethyl}-2-methoxyphenol and 6-Paradol. The combination of all this compound can be used for the treatment of SARS-CoV-2. It was found from literature that 6 paradol chemical compound has biologically significant beyond its medicinal value especially in the formulation of herbal and synthetic drugs. Cancer-battling antioxidants, flavonoids, have been found in relative abundance in Aframomum melegueta [46]. Flavonoids are commonly reported to possess anti-carcinogenic and anti-mutagenic effects (47) in which they interfere with the development of malignant tumors by inhibiting the expression of mutant genes, inactivating carcinogens and enzymes that are involved in the activation of pro-carcinogens, as well as activating enzymatic systems that are involved in the detoxification of xenobiotics [47,48]. Phytochemical investigations of Aframomum melegueta revealed the presence of (-)-buplerol, (-)-arctigenin, (E)- 14-hydroxy-15-norlabda-8(17), 12-dien- 16-al, labda-8(17),12-dien-15,16-dial,16-oxo-8(17),12(E)-labdadien-15-oic acid, 5-hydroxy-7-methoxy flavone and apigenin in the extract. Among the list, (-)-arctigenin and (-)-buplerol showed the capacity to trigger apoptosis in pancreatic cancer cells [49].

Plate 1: Aframomum melegueta Seed, Fruit pulp (Mesocarp), Leaf sheath and Stem
Source (Osuntokun et al,.2020).
The seeds of the Aframomum melegueta pepper have been proven to reverse such toxic effects. Its hepatoprotective effect is evaluated based on the changes in ALT, GSH, thiobarbituric acid reactive substances, TNF-α, IL-1β levels and activities of some enzymes such as caspase 3 and 9 [50].
Aframomum melegueta Compounds present in the extract that can be possibly used to treat SARS-CoV-2 (COVID-19) include 3-(S)-acetyl- 1-(4′-hydroxy-3′, 5′-dimethoxyphenyl)-7-(3″,4″,5″-trihydroxyphenyl) heptane and dihydrogingerenone that can suppress TNF-α and IL-1β levels in the body. These compounds impede the reduction of GSH by trapping free radicals arising from liver hepatocytes. In addition, these compounds also lower the levels of ALT, TNF-α, IL-1β as well as caspases 3 and 9. Apoptosis of hepatocytes induced by CCl4 is also inhibited. The hepatoprotective effect of Aframomum melegueta is due to its ability to suppress inflammatory responses and apoptosis, as well as the ability to forage free radicals [51].
Secondary metabolite (Phytochemical) of Aframomum melegueta investigations revealed the presence of (-)-buplerol, (-)-arctigenin, (E)- 14-hydroxy-15-norlabda-8(17), 12-dien-16-al, labda -8(17),12-dien-15,16-dial, 16-oxo-8(17),12(E)-labdadien-15-oicacid, 5-hydroxy-7-methoxy flav one and apigenin in the extract. Among the list, (-)-arctigenin and (-)-buplerol showed the capacity to trigger apoptosis in pancreatic cancer cells [52].
According to Vinegar et al, the development of the carrageenan induced inflammation is due to the release of cytoplasmic enzymes and serotonin from mast cells during hypersensitive type I reaction and the increase of prostaglandin release to the inflamed area [53]. While the anti-inflammatory activity of diclofenac is mediated chiefly through inhibition of the cyclooxygenase pathway (COX 1 and COX 2), particularly prostaglandins and that of dexamethasone is mediated through their suppressive effects on the inflammatory cytokines and on other lipid mediators of inflammation, It is however possible that the Aframomum melegueta extract may inhibit the release of inflammatory mediators released during hypersensitive type I reaction and carrageenan-induced inflammation [54]. The basis of the protective effect of Aframomum melegueta containing vitamin D is said to lie in its ability to stimulate innate immunity and to reduce inflammation. Innate immunity differs from adaptive immunity in that the former responds rapidly to microorganisms using genetically encoded effectors, most notably the antimicrobial peptides (AMPs) [52]. As noted, the active form of vitamin D (1,25-dihydroxyvitamin D; 1,25-OH2D) stimulates the expression of AMPs “endogenous antibiotics” in human monocytes, neutrophils, and epithelial cells [54]. AMPs protect epithelial surfaces by destroying the lipoprotein membranes of microbes such as influenza viruses. If the epithelial mucosal surface barrier is breached, microbes binding to the epithelia promote the expression of inducible AMPs including the defensins and cathelicidin. The defensins inhibit influenza hemagglutinin A-associated carbohydrates [53] and act with cathelicidin as chemoattractants for macrophages and neutrophils [54]. Vitamin D thus enhances the capacity of the epithelium to produce AMPs following exposure to microbes. Aframomum melegueta also dampens the proinflammatory peptides interferon gamma, TNF-alpha and IL-12 of the adaptive immune system, especially those responsible for acute inflammation “cytokine storms” [55]. This fact about Aframomum melegueta, makes it a choice medicinal plant for the treatment of SARS-CoV-2 (COVID-19), if it is scientifically explored especially during this SARS-CoV-2 (COVID-19) menace.
Spondias mombin belongs to the family of Anacardiaceae, one of the potent medicinal plant that has a very high medicinal value becomes a viral tools in the production of new and novel antimicrobial agent especially during this pandemic era of world history if medically explored because of its various therapeutically advantageous against infectious organism. This will help us to totally eradicate the recalcitrant infection like SARS-CoV-2 (COVID-19) and HIV, Breast cancer, liver infection just to mention a few [55].
Spondias mombin is a magic bullet against infectious diseases. Spondias mombin contains flavonoids also inhibit specific enzymes, which can block the angiotensin-converting enzyme (ACE) that raises blood pressure, which is one of the suppose symptom of SARS-CoV-2 (COVID-19). Spondias monbin extract act by blocking the “suicide” enzyme cyclooxygenase that breaks down prostaglandins, they prevent platelet stickiness and hence platelet aggregation [56].

Plate 2: Spondias mombin Tree with Developing Fruit and Leaf sheath.
Source Osuntokun et al,.2019 (55)
Spondias mombin contains Catechin and Epigalocatechin, Epicatechin, Stigmasterol, and Phytosterol, a very important flavonoid that can be used in drug formulation because of its extensive therapeutic properties. Catechins inhibites replication of influenza virus on MDCK cells. The inhibitory effect is in a concentration-dependent manner and throughout the virus infection cycle after initial infection. Antiviral effects exerted not only on initially infecting viruses but on newly propagated viruses like SARS-CoV-2 [57].
Epicatechin is a type of flavonoid which is mainly found in green tea (Camellia sinensis) and dark chocolate; it has now been discovered in Spondias mombin which is good alternative to green tea in places like West Africa where green tea is not found in large quantity [58]. Polyphenols comprise of 30-40% of extractable solids from dried Spondias mombin stem bark [58].
Epicatechins have been proven to have diverse benefits to human health and can serve as a predictive treatment of SARSCoV-2. Epicatechins can be used to reduce the risks of diabetes mellitus and cardiovascular diseases and in the treatment of SARS-CoV-2 Pharmalogical effect of Epicatechins content of Spondias mombin as well as antihyperlipidemic, anti-inflammatory, antioxidative, anticarcinogenic, and cytoprotective effect should be emphasized [59]. These flavonoids can be used as therapeutic agents individually or in combination with other synthetic drugs and antibiotics to produce a new generation of phytopharmaceuticals which can formulated in synthetic and herbal drug during this pandemic era of SARS-CoV-2 infection.
Natural product like epicatechin is cost effective, highly biocompatible and has low toxicity [60].Epicatechin (EC) helps in the inhibition of HIV-1 reverse transcriptase in vitro and binds directly to CD4 molecule with consequent inhibition of gp120 binding [61].
Epigallocatechin (EGC) inhibited plaque forming activity of influenza A and B virus whereas EC exhibited little inhibition. A polyphenol mixture is more efficient than a single compound in plaque inhibition and epicatechin had no antiviral effect and only a little inhibition on plaque formation [62]. (+)-Epicatechin and (-)-epicatechin was found to inhibit Hepatitis C virus (HCV) replication by inactivating Cyclooxgenase-2-depandant signalling pathway. Cyclooxygenase-2 (COX-2) produces various prostaglandins (PG) which result in chronic inflammation and fibrosis. PGE2, thromboxane B2 and prostacylin gives rise to cellular proliferation, cancer invasiveness, angiogenesis and antiapoptotic.
Therefore, leading to hepatocarcinogenesis, (+)-Epicatechin and (-)-epicatechin may suppress the gene expressions of pro-inflammatory enzymes and cytokines that are induced by HCV [63].
Epigallocatechin (EGC) increases lysosomal acidification, regulates autophagy and lipid clearance in liver due to its antisteatotic property [64]. EGC, in dose dependent manner can reduce the release of cytokines/chemokines responsible for inflammation showing anti-inflammatory property [65]. Its antiallergic property strongly inhibits activation of mast cells and expression of high-affinity IgE receptor, which produces an allergic reaction on exposure to certain foreign antigens [66].
The most important antioxidant property is very crucial in treating chronic diseases which are related to oxidative stress, cardiovascular, neurodegenerative diseases and cancer. Research on this property revealed information about its anticardiovascular and anti-hypertensive activity which enables epigallocatechin, epicatechin (EGC, EC) and polysteroids, to prevent platelet aggregation, lower cholesterol level and inhibit lipid peroxidation [67].
Epigallocatechin, Epicatechin (EGC, EC) and polysteroids is also beneficial for preventing aging of brain and other neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases as depicted from mouse model studies [68]. EGC and EC also show antihypercholesterolemic (anti-obesity) activity and promote weight loss through fat oxidation. Animal studies have demonstrated that EGC, EC and polysteroids is an efficient agent in preventing the development of diabetes, type 1 or type 2 [69]. EGC affects each step of the HIV life cycle, from cell attachment, entry of virus, replication cycle to the expression of mRNA. It also interferes with the infectivity of the virus by binding to the surface of viral envelope and deforming the phospholipids followed by lysis of the virus particle [70]. The attachment of the gp120 envelope protein to the CD4 receptor on T-helper cells initiates the entry of HIV-1 into the host. EGC blocks the interaction of gp120 and CD4 and prevents the attachment of HIV-1 virions, EGC also inhibits the viral production from infected cells and the level of expression of viral mRNA. EGC arts as a strong inhibitor of HIV replication in cultured peripheral blood cells and inhibition of HIV-1 reverse transcriptase in vitro.
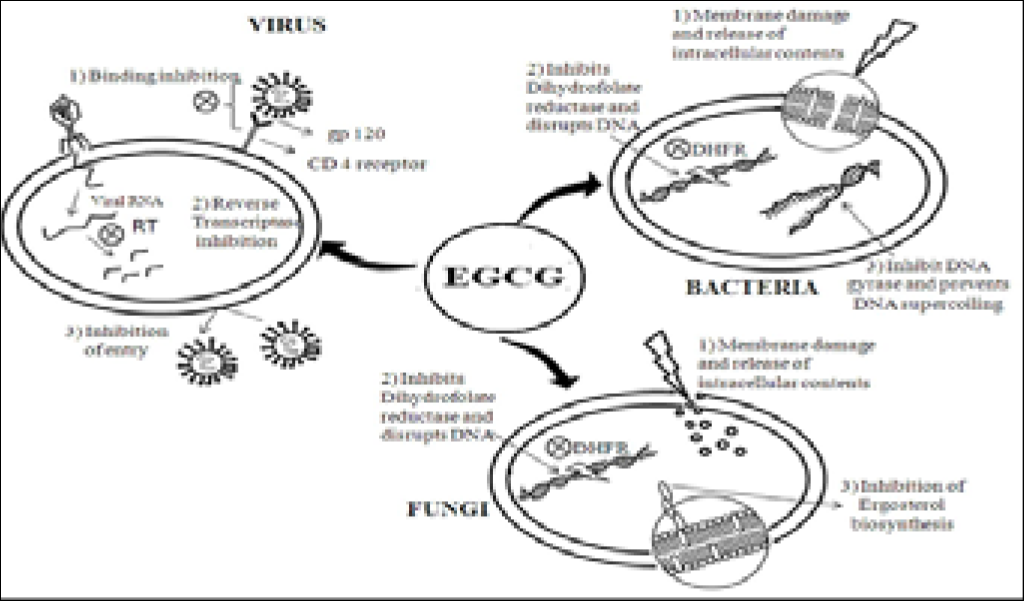
Schematic Diagram of mechanisms of action Epigallocatechin and Epicatechin (EGC, EC)
EGC binds directly to CD4 molecules with consequent inhibition of Gp 120 binding and inactivate viruses in vitro by deformation of phospholipids. It should be mentioned that EC are antioxidant, they are effective scavengers and free radical such a reactive oxygen species (ROS), Reactive nitrogen species and superoxide [71].
Epicatechin have been proven to have diverse benefits to human health. It reduces the risks of diabetes mellitus and cardiovascular diseases this shows the basic important aspect of Spondias mombin tree, which have pharmalogical effects such as anti hyporlipidemic, anti-inflammatory, antioxidative effect, anticarcinogenic and cytoprotective. Epicatechin and other polyphenols decreases the susceptibility of low density lipoprotein to oxidation which prevents the initiation of artherosclerosis, HIV protein (Tat) and gp120 is known to cause neurotoxicity in human via mechanisms that activate macrophages and glial cells and finally, oxidative stress it can be suggested that epicatechin are neuroprotective by blocking the neurotoxic effects of the HIV protein which cause oxidative stress [71].
In conclusion, coronavirus disease (COVID-19) pandemic infection should not be a death sentence; it should be an advancement into the scientific world especially the naturally occurring product that can be used in the formulation of new antimicrobial agents. Little is known of this two plants, but their uses, is limitless and it should be explored especially during this time of need which viral infection of COVID 19 becomes a menace
- Naming the coronavirus disease (COVID-19) and the virus that causes it”. World Health Organization (WHO). Archived from the original on 28 February 2020. Retrieved 28 February 2020.
- WHO Director-General’s opening remarks at the media briefing on COVID-19”. World Health Organization (WHO) (Press release). 11 March 2020. Archived from the original on 11 March 2020. Retrieved 12 March 2020.
- Hopkins Claire (2020) “Loss of sense of smell as marker of COVID-19 infection”. Ear, Nose and Throat surgery body of United Kingdom. Retrieved 28 March 2020.
- Velavan TP, Meyer CG (2020) “The COVID-19 epidemic”. Tropical Medicine & International Health. n/a (n/a): 278-280. doi:10.1111/tmi.13383.
- Symptoms of Coronavirus”. U.S. Centers for Disease Control and Prevention (CDC). 10 February 2020. Archived from the original on 30 January 2020.
- Q&A on coronaviruses”. World Health Organization (WHO). Archived from the original on 20 January 2020. Retrieved 27 January 2020.
- How COVID-19 Spreads”. Centers for Disease Control and Prevention (CDC). 2 April 2020. Archived from the original on 3 April 2020. Retrieved 3 April 2020.
- Q & A on COVID-19”. European Centre for Disease Prevention and Control. Archived from the original on 5 February 2020. Retrieved 23 March 2020
- Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations”. World Health Organization. 29 March 2020. Retrieved 3 April 2020. According to current evidence, COVID-19 virus is primarily transmitted between people through respiratory droplets and contact routes
- Organization (WHO), World Health (28 March 2020). “FACT: #COVID19 is NOT airborne. The #coronavirus is mainly transmitted through droplets generated when an infected person coughs, sneezes or speaks.To protect yourself:-keep 1m distance from others-disinfect surfaces frequently-wash/rub your -avoid touching your pic.twitter. com/fpkcpHAJx7”. @WHO. Retrieved 3 April 2020. These droplets are too heavy to hang in the air. They quickly fall on floors or surfaces.
- New coronavirus stable for hours on surfaces”. National Institutes of Health. 17 March 2020. Archived from the original on 23 March 2020. Retrieved 23 March 2020.
- Coronavirus disease 2019 (COVID-19) Situation Report—73”(PDF). World Health Organization. 2 April 2020. Retrieved 3 April 2020
- Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19)”. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 4 March 2020. Retrieved 26 March 2020.
- Salehi Sana, Abedi Aidin, Balakrishnan Sudheer, Gholamrezanezhad Ali (2020) “Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients”. American Journal of Roentgenology: 1–7. Doi 10.2214/AJR.20.23034. ISSN 0361-803X. PMID 32174129.
- ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection”. American College of Radiology. 22 March 2020. Archived from the original on 28 March 2020.
- How to Protect Yourself & Others”. Centers for Disease Control and Prevention (CDC). 8 April 2020. Archived from the original on 26 February 2020. Retrieved 9 April 2020
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (2020) “Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study”. Lancet 395 (10223): 507– 513. doi:10.1016/S0140-6736(20)30211-7
- Hessen MT (2020) “Novel Coronavirus Information Center: Expert guidance and commentary” Elsevier Connect. Archived from the original on 30 January 2020. Retrieved 31 January 2020.
- Lai Chih-Cheng, Shih Tzu-Ping, Ko Wen-Chien, Tang Hung-Jen, Hsueh Po-Ren (2020) “Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges”. International Journal of Antimicrobial Agents 55 (3): 105924. doi:10.1016/j.ijantimicag.2020.105924. ISSN0924-8579 PMID 32081636
- World Health Organization (19 February 2020). “Coronavirus disease 2019 (COVID-19): situation report, 29”. World Health Organization (WHO). hdl:10665/331118.
- Bourouiba L (2020) “Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19”. JAMA. doi:10.1001 /jama.2020.4756. PMID 32215590.
- Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations”. www. who.int. Retrieved 29 March 2020.
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. (2020) “Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1”. New England Journal of Medicine: NEJMc2004973. doi:10.1056/ NEJMc20 04973. ISSN 0028-4793. PMC 7121658. PMID 32182409.
- Moriyama M, Hugentobler WJ, Iwasaki A (2020) “Seasonality of Respiratory Viral Infections”. Annual Review of Virology. 7. doi10.1146/annurevvirology-012420-022445. PMID 32196426.
- COVID-19 prevention: Why soap, sanitizer and warm water work against coronavirus—CNN”. Edition.cnn. com. 24 March 2020. Retrieved 7 April 2020
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (2020) “The proximal origin of SARS-CoV-2”. Nature Medicine: 1–3. doi:10.1038/s41591-020-0820-9.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. (2020) “A Novel Coronavirus from Patients with Pneumonia in China, 2019”The New England Journal of Medicine. 382 (8): 727–733. doi:10.1056/NEJMoa2001017. PMC 7092803. PMID 31978945.
- Cyranoski D (2020) “Mystery deepens over animal source of coronavirus”. Nature. 579 (7797): 18–19. Bibcode:2020Natur. 579…18C. doi:10.1 038/d4 1586-020-00548-w. PMID32127703.
- Letko M, Marzi A, Munster V (2020) “Functional assessment of cell entry and receptor usage for SARSCoV-2 and other lineage B betacoronaviruses”. Nature Microbiology 5(4): 562–569. doi:10.1038/s41564-020-0688-y. PMID 32094589.
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X (2020) “High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa”. International Journal of Oral Science 12(1): 8. doi:10.1038/s41368-020-0074-x. PMC 7039956. PMID 32094336
- Gurwitz D (2020) “Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics”. Drug Development Research. doi:10.1002/ ddr.216 56 PMID 321 9518.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, Goor H van (2004) “Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis”. The Journal of Pathology 203 (2): 631–637. doi:10.1002/path.1570. ISSN 1096-9896. PMID 151 413 77.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (2020) “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China”. Lancet 395(10223): 497–506. doi:10.1016/S0140-6736(20)30183-5 PMID 31986264.
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y (2020) “Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus”. bioRxiv Pre-print. doi:10.1101/2020.02.12.945576.
- Centers for Disease Control (2020) “Coronavirus Disease 2019 (COVID-19): Prevention & Treatment”. Archived from the original on 15 December 2019. Retrieved 10 February 2020.
- World Health Organization “Advice for Public”. Archived from the original on 26 January 2020. Retrieved 10 February 2020.
- Fisher D, Heymann D (2020) “Q&A: The novel coronavirus outbreak causing COVID-19”. BMCMedicine. 18 (1):57. doi:10.1186/s12916-020-01533-w. PMC7047369 . PMID 321 06852.
- Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. (2020) “Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province”. Chinese Medical Journal: 1. doi:10.1097/CM9.00 00000000000744. P MID 32044814.
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (PDF) (Report). World Health Organization (WHO). 16–24 February 2020. Archived (PDF) from the original on 29 February 2020. Retrieved 21 March 2
- Li G, De Clercq E (2020) “Therapeutic options for the 2019 novel coronavirus (2019-nCoV)”. Nature Reviews. Drug Discovery 19(3): 149–150. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- Kupferschmidt Kai, Cohen Jon (2020) “WHO launches global megatrial of the four most promising coronavirus treatments”. Science Magazine. doi:10.1126/sci ence. abb8497. Retrieved 27 March 2020
- Beeching NJ, Fletcher TE, Fowler R (2020) “BMJ Best Practices: COVID-19”(PDF). BMJ. Archived (PDF) from the original on 22 February 2020. Retrieved 11 March 2020.
- Gao J, Tian Z, Yang X (2020) “Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies”. Bioscience Trends 14 (1): 72–73. doi:10.5582/ bst.2020.01047. PMID 32074550.
- Touret F, de Lamballerie X (2020) “Of chloroquine and COVID-19”. Antiviral Research. 177: 104762. doi:10.1016/j.antiviral .2020.104762.
- Multicenter collaboration group of Department of Science Technology of Guangdong Province Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia (2020). “[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]”. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chinese Journal of Tuberculosis and Respiratory Diseases. 43: E019. doi:10.3 760/cma.j.issn.1001-0939.2020.0019. PMID 32075365
- Doherty VF, Olaniran OO, Kanife UC (2010) Antimicrobial Activities of AframomumMelegueta (Alligator Pepper). International Journal of Biology. Vol 2(2): 126-131.
- Yamaguchi K, Honda M, Ikigai h, Hara Y, Shimamura T (2002) Inhibitory effects of (-)-Epi gallocatechingallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antivir Res 53: 19-34.
- Del Bo C, Bernardi S, Marino M, Porrini M, Tucci M, Guglielmetti S, Cherubini A, Carrieri, B, Kirkup B, Kroon P (2019) Systematic Review on Polyphenol Intake and Health Outcomes: Is there Su_cient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 11: 1355.
- Lin YT, Wu YH, Tseng CK, Lin CK, Chen WC, Hsu YC, Lee JC (2013) Green tea phenolic epicatechins inhibit Hepatitis C virus replication via cyclooxygenase-2 and attenuate virus-induced inflammation. PLoS ONE 8(1): e54466
- El Halawany AM, El Dine RS, El Sayed NS, Hattori M (2014) Protective effect of Aframomum melegueta phenolics against CCl4-induced rat hepatocytes damage: Role of apoptosis and pro-inflammatory cytokines inhibition. Scientific Reports 4: 5880.
- Oludare Temitope Osuntokun, Baraldi Cecilia, Gamberini Maria Cristina (2018) Evaluation of Quantitative Elemental Compositions and Antioxidant Potentials of Spondias mombin Extracts (Linn), A Precursor Against InfectiousDiseases,World Journal of Pharmacy and Pharmaceutical Sciences 7(3): 964-985.
- Oludare Temitope Osuntokun(2020) Aframomum melegueta (Grains of Paradise) Annals of Microbiology and Infectious Diseases 3(1): 1-6.
- Martinez SE, Davies NM, Reynolds JK (2013) “Toxicology and Safety of Flavo noids”. Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology. John Wiley & Son. p. 257. ISBN 978-0-470-57871-1.
- Wagner H, Ulrich-Merzenich G (2009) Synergy research: approaching a new generation of phytopharmaceu ticals. Phytomedicine 16: 97–110.
- Oludare Temitope Osuntokun,V. O. Olumekun, A. O. Ajayi, I. O. Omotuyi and A. Olonisakin (2020)Assessment of in-vitro Antioxidant/Enzymes Inhibitory Potentials of Aframomum melegueta[Roscoe]K.Schum(Grains of Paradise)Leaf, Stem Bark, Seed Bark and Seed Extracts, Archives of Current Research International 20(2): 40-57, 2020 Article no.ACRI.55957 ISSN: 2454-7077,Pp40-57
- Temitope OO, Ogunmodede AF, Fasusi OA, Thonda AO, Odufunwa AE (2017) Synergistic antibacterial and antifungal activities of Spondias mombin extracts and conventional antibiotic and antifungal agents on selected clinical microorganisms. Sch J App Med Sci 5: 307-318.
- Nelson CJ (2005) Composition of the leaf oils of four 2. Spondias species from Brazil. Journal of Essential Oil Research 7: 561-563.
- Oludare temitope Osuntokun, AO, Oluduro, TO Idowu & AO Omotuyi (2017)Assessment of Nephrotoxicity, Anti-inflammatory and Antioxidant properties of Epigallocatechin, Epicatechin and Stigmasterol phytosterol (synergy) Derived from ethyl acetate stem bark extract of Spo ndias mombin on Wister Rats Using Molecular method of analysis, Journal of Molecular Micro biology 1(1): 1-11.
- Osuntokun OT, Idowu TO, Cristina GM (2018) Bio-guided Isolation, 16. Purification and Chemical Characterization of Epigallocatechin; Epi¬catechin, Stigmasterol Phytosterol from of Ethyl Acetate Stem Bark Fraction of Spondias mombin (Linn.) Bio chem Pharmacol (Los Angel,USA) 7: 1-9.
- Moronkola DO, Adeleke AK, Ekundayo O (2003) Constituents of the 9. Spondias mombin Linn and the comparison between its fruit and leaf essential oils. Journal of Essential Oil Bearing Plants 6: 148-152.
- Ayoka AO, Akomolafe RO, Iwalewa EO, Akanma MA, Ukponmwan 11. OE (2006) Sedative,epileptic and antipsychotic effects of Spondias mombin L (Anacardiaceae ) in mice and rats. Journal of Ethnophar¬macolology 103: 166-175.
- Oboh G, Rocha JBT (2007) Polyphenol in red pepper [Capsicum annuum Var. aviculare (Tepin) and their protective effect on some prooxidants induced lipid peroxidation in brain and liver. Eur. Food Res. Technol 225(2): 239-247.
- Tachibana H. Green tea polyphenol sensing. Proc (2011) Jpn Acad Ser B Phys Biol Sci 87: 66-80.
- Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH (2014) Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS One 9: e87161.
- Parmar N, Rawat M, Kumar VJ. Camellia Sinensis (Green Tea) (2012): A Review. Glob J of Pharmacol 6: 52-59.
- Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional signi®cance. Nutr Rev 56: 317-333.
- Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH (2011) (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol 589(18): 4615-4631
- Quinde-Axtell Zory, Baik Byung-Kee (2006) “Phenolic Compounds of Barley Grain and Their Implication in
Food Product Discoloration”. J. Agric. Food Chem 54(26): 9978–9984. doi:10.1021/jf060974w. PMID 17177530. - Arunachalam M, Mohan N, Sugadev R, Chellappan P, Mahadevan A (2003) “Degradation of (+)-catechin by Acinetobacter calcoaceticus MTC 127”. Biochimica et Biophysica Acta (BBA) – General Subjects 1621(3): 261– 265. doi:10.1016/S0304-4165(03)00077-1.
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Hori N, Watanabe T, T akahashi K, Nagawa H(2003) Epigallocatechingallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J Allergy ClinImmunol 112: 951-957.
- Oludare Temitope Osuntokun, TO Idowu, Gamberini Maria Cristina (2018) Bio-guided Isolation,Purification and Chemical Characterization of Epigallocatechin; Epicatechin, Stigmasterol Phytosterol from of Ethyl Acetate Stem Bark Fraction of Spondias mombin(Linn.) BiochemPharmacol (Los Angel) 7:240.doi: 10.4172/2167 0501 .1000240 7(1): 1-9.













