Article / Research Article
1Community Rehabilitation Service Support Center, Hospital Authority, Hong Kong
King-Pong Yu
Community Rehabilitation Service Support Center,
Hospital Authority,
Hong Kong
15 August 2023 ; 16 September 2023
Home Hemodialysis(HD) is an advantageous alternative to in-center treatments, offering greater convenience, flexibility, and improved quality of life [1]. The rising prevalence of end-stage renal disease (ESRD) globally has increased the demand for accessible renal replacement therapies [2]. Factors such as an aging population, higher rates of chronic conditions, improved survival rates for chronic kidney disease patients, and increased healthcare access contribute to the growing burden on healthcare systems [3]. Home HD has gained prominence by enabling patients to receive treatment at home, providing control over their healthcare management [1]. It offers flexibility in scheduling, reduces travel time and costs, and creates a relaxed environment that positively impacts the patient experience [4]. Improved clinical outcomes, including better blood pressure control and enhanced quality of life, have been associated with home HD. However, challenges persist during the inlet/ outlet preparation and tubing connection phase, involving awkward elbow and wrist maneuvers, which can cause joint stress and discomfort [5]. Limited mobility or dexterity can further complicate the task, leading to potential complications. Addressing these challenges and promoting patient autonomy in tubing connection are crucial for improved treatment experiences and outcomes. Innovative solutions are being developed to facilitate independent tubing connection in home HD, empowering patients and reducing complications. Evaluations of the aids have shown promising results, enhancing patient outcomes and satisfaction [6].
This article innovates 3D printed aids called needle and tubing connection holder (NATCH) that promote independent tubing connection in home HD, reviewing challenges, discussing device development and fabrication. Implications for patients, caregivers, and healthcare providers are discussed, emphasizing the need for further research and innovative devices to enhance patient autonomy. By addressing these challenges and promoting independent self-care, home HD patients can experience improved quality of life, reduced caregiver burden, enhanced treatment outcomes and providing insights for healthcare professionals, researchers, and policymakers to support patient autonomy in home HD.
3D Scanning
The 3D scanning procedure holds significant importance in accurately capturing the precise shape and dimensions of the patient’s arm, thereby ensuring an optimal fit for the aid. The scanning process commences with the meticulous preparation of the patient’s arm, which is positioned in a comfortable and straight manner, simulating the conditions during HD procedures, where an arm splint will be provided during HD, to stabilize the elbow preventing any movement that may compromise the stability of the intravenous drip or risk damaging blood vessels.
To achieve a compromise level of accuracy, minimizing movement of the patient’s arm during the scanning process becomes crucial. A handheld 3D scanner (Shining 3D Tech. Co., Ltd. Hangzhou, China) is utilized to capture the intricate contour of the patient’s arm (figure 1). The scanner calculates intricate details such as distances and depth information, ultimately generating a highly precise 3D point cloud representation of the arm. Throughout the scanning process, the rehabilitation engineer skillfully maneuvers the scanner around the arm, capturing multiple viewpoints and perspectives. This comprehensive approach ensures the capture of the arm’s complete anatomical shape and contours from diverse angles. The rehabilitation engineer may need to fine-tune the scanner’s settings, such as resolution, exposure and brightness, to accommodate the intricacies of the arm’s shape and the desired level of detail. Therefore, a real-time feedback capability is essential, to visualize and assess the scanned data on a computer screen.
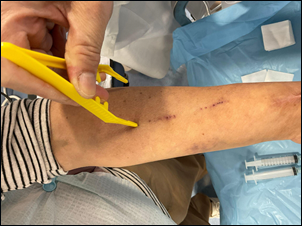 Figure 1: Arm of the haemodialysis patient. The responsible renal nurse is indicating the appropriate location for needle injection.
Figure 1: Arm of the haemodialysis patient. The responsible renal nurse is indicating the appropriate location for needle injection.
Maintaining a meticulous focus on the scanning process entails ensuring comprehensive coverage of all relevant areas of the arm, including the specific regions such as the transplanted vein where the aid will be positioned. Upon the completion of the scanning phase, the collected data is subjected to sophisticated 3D scanning software for processing, where the software employs the acquired data to create a high-resolution 3D model of the patient’s arm. (figure 2).
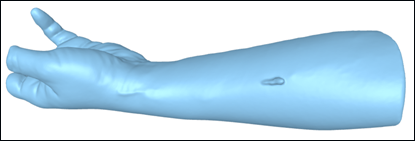 Figure 2: 3D scanned mesh model of the patient’s arm. The model was collected to mold a drip mount according to the dimensions of the arm.
Figure 2: 3D scanned mesh model of the patient’s arm. The model was collected to mold a drip mount according to the dimensions of the arm.
3D Modelling
Occupational therapist and rehabilitation engineers at Community Rehabilitation Service Support Center (CRSSC), utilize computer-aided design (CAD) software (SolidWorks, version 2011; Dassault Systemes, Velizy, France) to design a lock head component (figure 3), serving as the central unit of the NATCH with the capability to securely retain the supercath and facilitate the removal of cover through rotational manipulation during tubing connection.
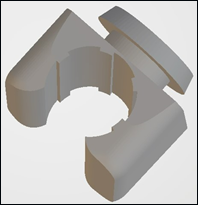
 Figure 4: Mesh models of the lock-head component and the 3D scanned arm. Rehabilitation engineers engaged in the process of aids modeling based on the contour of the 3D scanned arm.
Figure 4: Mesh models of the lock-head component and the 3D scanned arm. Rehabilitation engineers engaged in the process of aids modeling based on the contour of the 3D scanned arm.
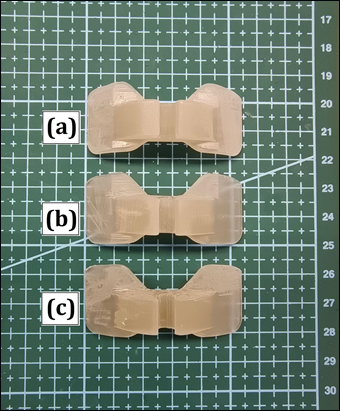 Figure 5: Comparison of the 3D printed components after different sterilization procedures: (a) no sterilization; (b) 30 minutes sterilization and (c) 60 minutes sterilization. There was no significant wear, cracks or breakage found, indicating the possibility of sterilization of the aids before each usage.
Figure 5: Comparison of the 3D printed components after different sterilization procedures: (a) no sterilization; (b) 30 minutes sterilization and (c) 60 minutes sterilization. There was no significant wear, cracks or breakage found, indicating the possibility of sterilization of the aids before each usage.
NATCH was fabricated utilizing Form 3B+(Formlabs, Massachusetts, USA) (figure 6), printing configuration are listed in table 1. The digital CAD model, created based on the 3D-scanned contour of the patient’s arm, served as the foundation for the printing process. Layer by layer, the 3D printer deposited the skin-safe SLA resin material, gradually constructing the aid models with exceptional precision and accuracy.
 Figure 6: Operation of the SLA 3D printer Form3B+. The final model was designed and sent to the 3D printer to proceed on fabrications.
Figure 6: Operation of the SLA 3D printer Form3B+. The final model was designed and sent to the 3D printer to proceed on fabrications.
| 3D Printing parameters / valuese | |
| Machine | Form 3B+ |
| Material | Tough 1500 |
| Layer height [mm] | 0.1 |
| Alcohol wash(machine model/ Agitation method) | Form Wash / Magnetically coupled impeller |
| Washing settings(duration [hr]/ temperature [°C]) | 1.0 / NA |
| UV curer (machine model/ LED wavelength / power [W]) | Form Cure / 405nm / 39W |
| Curing setting (duration [hr]/ temperature [°C]) | 1.0 / NA |
| Printing (duration [hr]/volume [ml] ) | 2.25/7.57 |
Table 1: Printing Configuration of the NATCH
Post-Processing
Post-processing steps were carried out in accordance with the recommendations outlined in publications [9] [10], despite their distinct origins but similar approach, to refine and prepare the NATCH for utilization. Initially, the models underwent an alcohol rinse process using an alcohol washer – Form Wash (Formlabs, Massachusetts, USA) and 95% isopropyl alcohol (One Stop Medical Limited, Hong Kong) to effectively eliminate any uncured resin residual, thereby ensuring a surface that is characterized by cleanliness and hygiene. Subsequently, the models were subjected to exposure to 405nm wavelength, 39W power UV light provided by a resin UV curer – Form Cure (Formlabs, Massachusetts, USA), a widely employed technique for the curing of SLA resin. This process served to enhance the material’s strength and improve its structural integrity. To optimize patient comfort, a final step involving surface finishing was conducted. Subsequently, the support structure was carefully eliminated using an ultrasonic cutter – Phrozen Sonic Saber (Phrozen Tech Co., Ltd., Hsinchu, Taiwan) to safeguard the structural integrity of the model. This procedure involved the utilization of waterproof abrasive paper (Riken Corundum Carbosand waterproof paper cc600; Riken Co, Tokyo, Japan) to attain a refined surface texture, effectively eliminating any rough edges or imperfections present on the models. As a result, this comprehensive surface refinement process contributed to a comfortable fit and substantially diminished the probability of irritation or discomfort during extended periods of use.
Patient Trial
To assess the usability and effectiveness of the NATCH, a patient trial phase was conducted. Initially, the aid prototypes were tested at clinics under the supervision of renal nurses, occupational therapists, and rehabilitation engineers (figure 7). Feedback from both patients and healthcare professionals was collected to evaluate the NATCH’s comfort, convenience, and overall functionality. Following the clinic trial, the NATCH was provided to patients for a trial period in their own homes (figure 8). During the practical testing phase, the tele-rehabilitation method using video conference technology was adopted to ensure patient safety and proper adherence to the prescribed procedures by renal nurses [11]. This approach facilitated remote monitoring, guidance, and correction of the patients’ usage of the NATCH, promoting safety and accuracy in their application.
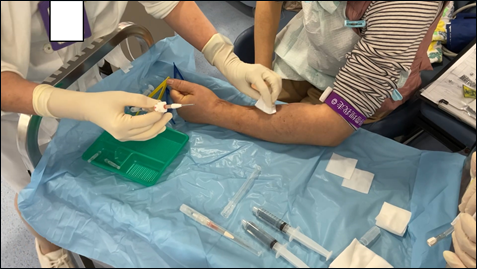 Figure 7: Renal nurse engaged in the aids trial on the patient.
Figure 7: Renal nurse engaged in the aids trial on the patient.
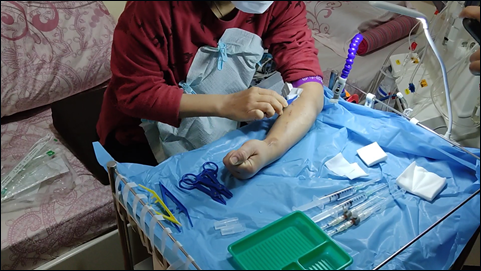 Figure 8: The patient used the aids at home for the first time while adopting tele-rehabilitation methods to ensure safety of usage and compliance of prescribed procedure.
Figure 8: The patient used the aids at home for the first time while adopting tele-rehabilitation methods to ensure safety of usage and compliance of prescribed procedure.
This study has an inherent limitation related to its necessity for continuous placement on the patient’s arm throughout the extended duration of hemodialysis, typically lasting at least five hours. This requirement becomes particularly challenging due to the weight of the NATCH, which increases the probability of unintentional displacement of the NATCH. This dislodgement poses a significant concern as it can negatively impact the stability and integrity of the supercath needle, subsequently compromising the safety of the HD procedure.
Addressing this limitation requires the development of innovative solutions to enhance device stability and minimize the susceptibility to displacement. This could involve the exploration of advanced securing mechanisms, novel materials, or ergonomic designs that optimize patient comfort while ensuring the device remains firmly in place. Further research and development endeavors are required to address this limitation and advance the reliability and efficacy of the NATCH.
In conclusion, the NATCH developed for home HD has revolutionized patient care, particularly for individuals who lack a caregiver at home. The advent of the NATCH has alleviated the burden of frequent visits to renal clinics, enabling patients to perform HD conveniently in the comfort of their own homes, and even on a daily basis. This flexibility empowers patients to integrate their treatment seamlessly into their lives, as they can undergo HDat night while maintaining their daily routines during the day.
The impact of the NATCH on patient quality of life cannot be underestimated. Patients now have the freedom to engage in social activities, such as going out for lunch with friends or pursuing employment, without compromising. This aid enhances their overall well-being and contributes to a higher quality of life. Moreover, the ability to conduct HD more frequently, as opposed to the traditional two-day cycle, offers potential health benefits, such as improved fluid and waste management, leading to better outcomes and overall health for the patients.
The author affirms the work’s originality and confirms that consent was obtained for the inclusion of the patient’s photograph.
No external funding was received for the research and publication of this study.
- Jaber, B. L., Lee, Y., Collins, A. J., Hull, A. R., Kraus, M. A., McCarthy, J., … & FREEDOM Study Group. (2010). Effect of daily HDon depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. American Journal of Kidney Diseases, 56(3), 531-539.
- Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., … & Yang, C. W. (2013). Chronic kidney disease: global dimension and perspectives. The Lancet, 382(9888), 260-272.
- Vos, T., Allen, C., Arora, M., Barber, R. M., Bhutta, Z. A., Brown, A., … & Boufous, S. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The lancet, 388(10053), 1545-1602.
- Lok, C. E., Sontrop, J. M., Tomlinson, G., Rajan, D., Cattral, M., Oreopoulos, G., … & Moist, L. (2013). Cumulative patency of contemporary fistulas versus grafts (2000–2010). Clinical journal of the American Society of Nephrology: CJASN, 8(5), 810.
- Wallace, E. L., Rosner, M. H., Alscher, M. D., Schmitt, C. P., Jain, A., Tentori, F., … & Sloand, J. A. (2017). Remote patient management for home dialysis patients. Kidney international reports, 2(6), 1009-1017.
- Chan, S. F., Ng, M. L., Chan, S. Y., Chan, M. M. L., Lau, W. Y., Chan, K. K., & Pong, Y. K. (2019). 44. A third hand for home haemodialysis. Renal Society of Australasia Journal, 15, 35-35.
- Said, N., Lau, W. J., Ho, Y. C., Lim, S. K., Zainol Abidin, M. N., & Ismail, A. F. (2021). A review of commercial developments and recent laboratory research of dialyzers and membranes for HDapplication. Membranes, 11(10), 767.
- Park, S. Y., Kang, S., Park, J. M., An, H. J., Oh, D. H., & Kim, J. I. (2019). Development and dosimetric assessment of a patient-specific elastic skin applicator for high-dose-rate brachytherapy. Brachytherapy, 18(2), 224-232.
- Kim, H. C., & Lee, S. H. (2005). Reduction of post-processing for stereolithography systems by fabrication-direction optimization. Computer-Aided Design, 37(7), 711-725.
- Koh, G. C. H., Yen, S. C., Tay, A., Cheong, A., Ng, Y. S., De Silva, D. A., … & Hoenig, H. (2015). Singapore Tele-technology Aided Rehabilitation in Stroke (STARS) trial: protocol of a randomized clinical trial on tele-rehabilitation for stroke patients. BMC neurology, 15(1), 1-14.













