Article / Short Communication
K K University, Nalanda, Bihar, India
Swapnila Roy
K K University
Nalanda
Bihar
India
10 September 2020 ; 9 October 2020
This paper is focused on the relationship between ozone depletion and environmental climate change. Ozone (O3) depletion and global warming are not directly related to each other but have a common reason as pollutants released into the atmosphere by human activities which alter both phenomenal change. Global warming is incident of accumulation of higher level of carbon dioxide into the atmosphere when hydrocarbons are used to generate electricity to run vehicles. Carbon dioxide spreads around the earth like a cover which is mainly responsible for the absorption of infrared radiation as a heat. Ozone depletion occurs when chlorofluorocarbons (CFCs) and halon (halogen) gases are observed in aerosol. Practically, spray cans and refrigerants are the sources of CFCs. Ozone is available in the stratosphere and absorbs ultraviolet radiaton, which is very harmful to humans, animals and plants. By photochemical reaction ozone molecules are broken down by CFCs and halons, which are the primary substances in the chemical reactions, reducing ozone’s ultraviolet radiation-absorbing capacity.
Keywords : Ozone Depletion, UV Radiation, CFC, Halon, Global Warming
There are several layers in Earth’s atmosphere. Among the different layers, the lowest layer of the atmosphere is closest to the Earth which is called troposphere. The range of the troposphere is from the surface up to about 10 km in altitude, which varies with latitude. In the troposphere temperatures decreases with altitude [1]. According to the rule of convection process there are huge air movements which mix the troposphere very efficiently. When ozone comes in contact with chlorine and bromine atoms in the stratosphere, they destroy ozone molecules. According to the photochemical reaction, the capacity of one chlorine atom to degrade 1 lakh ozone molecules before removing from the stratosphere. The destruction of Ozone [2] occur more quickly than it is naturally created. In the stratosphere, few compounds release chlorine or bromine in the exposure of intense UV light. Some chemical compounds are main contributor for ozone depletion which are called ozone-depleting substance (ODS) [3]. These compounds contribute to stratospheric ozone depletion [4]. ODS include chlorofluorocarbons (CFCs), hydro chlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, hydro bromofluorocarbons, chlorobromomethane, and methyl chloroform. In the troposphere the stability of ODS are very high which can be destroyed in the presence of strong ultraviolet light in the stratosphere. After degradation, chlorine or bromine atoms are released. Bromofluorocarbons are the compounds which contain bromine, fluorine, and carbon. The application of these compounds cause ozone depletion. The chlorofluorocarbons gases used for refrigeration, air conditioning. ODS release chlorine include chlorofluorocarbons which is used indifferent purposes. Since they are not destroyed in the lower atmosphere, so the drifting of CFCs [5] into the upper atmosphere within suitable conditions, ozone (O3) is destroyed by it. The absorption of part of sun radiation by ozone layer in the stratosphere, preventing it from reaching the earth’s surface. Significantly, it absorbs the portion of UV light called UVB. UVB has many harmful effects, including skin cancers, cataracts, and harm to some crops and marine life. For the production of chlorofluorocarbons (CFCs), carbon tetrachloride is widely used as a raw material which consisting of one carbon atom and four chlorine atoms, having many industrial uses. Overall, global production of CFCs and other ODS continued to use in domestic purpose earlier which were found in refrigeration, fire suppression, foam insulation, and other applications. The objective of this paper is to focus on the way by which ozone depletion facilitate the global environment.
The emission of solar electromagnetic radiation at various wavelengths, implies the energy at different intensities. The multi layered atmosphere signifies as a multi-layer shield which protects earth from harmful solar radiation [6]. Ground level ozone is a human health irritant and component of smog which is available in the lower atmosphere (troposphere) and has nothing to do with the ozone hole. High level ozone mostly available in the stratosphere which accounts for major ozone in atmosphere. The absorption of ultraviolet (UV) radiation by stratospheric ozone layer prevents living organisms from harmful UV rays without hitting Earth’s surface. UV rays are invisible to human but it is very powerful. UV radiation act partly as a substance of global warming [7] as its quantity of radiation is not as much as to trap excess heat in the atmosphere. UV radiation signifies a small part of the solar energy and comparing with other wavelength such as infrared wavelength, it is not highly absorbed or scattered in the atmosphere. Above all, the phenomena of ozone depletion are causing anxiety as it is health hazardous for human and all animals.
Chemically, an ozone molecule (O3) consists of three atoms of oxygen. In the stratosphere, ozone is referred to as the ozone layer which protects living organism on Earth by absorbing most of the ultraviolet (UV) radiation emitted by the sun [8]. Consequences of too much exposure of UV radiation resulted in skin cancer, cataracts, and depression of the immune system and also affects the productivity of certain crops. Scientifically, the stratospheric ozone is considered as good ozone. [Fig.1] On the other hand, artificially man-made ozone pollution affects at the ground level which is considered as bad ozone. This is a prime component of smog. Depletion of ozone layer started when man-made CFC molecules enrich the stratosphere level and are broken apart by short-wave energy from the sun. After transforming to free chlorine atoms by chemical reaction, these break apart molecules of ozone and create a hole in the ozone layer. The amount of CFCs in the stratosphere is now peaking. It’s great news that recently scientists expect that the ozone layer will be back to its previous stable size by the middle of the 21st century.
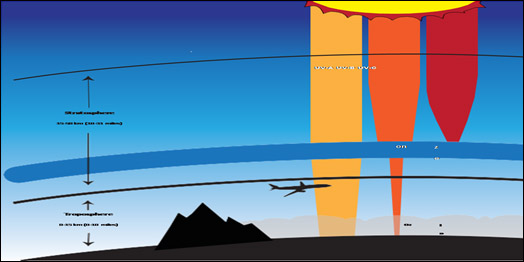
Figure 1: Ozone is good in stratosphere as it absorbs all of the most energetic ultraviolet radiation (UV-C), most of the UV-B radiation and least energetic UV-A radiation. In the troposphere it is harmful to breathe and primary component of smog in summer.
The first detection of ozone hole was an emotional debate in U S where all the industries fiercely resisted a ban on CFCs. The Ozone Hole [9] is not actual hole but the depletion of the ozone layer. The term ozone hole signifies the depletion of the protective ozone layer in stratosphere over Earth’s polar regions. All the living organism under the ozone hole are destroyed by the solar radiation while reaching the Earth’s surface, eventually it causes from eye damage to skin cancer. By the photochemical reaction, due to the action of the sun’s ultraviolet radiation on oxygen molecules stratospheric ozone is constantly formed. In the stratosphere below -78°C, thin clouds made of mixtures of ice, nitric acid, and sulfuric acid form. The chlorine-containing compounds like HCl is transformed from the reactants on the surfaces of these ice crystals which is harmless to ozone into more reactive forms. During sunrise over the Antarctic in the Spring (September), sunlight releases free chlorine atoms into the stratosphere. Then ozone destroying cycle started. After the chemical reaction between the chlorine atoms with ozone, CLO is formed. The CLO molecules combine with each other to form a dimmer. Chlorine atoms released by sunlight from the dimer, and the cycle restarted. The ozone-depleted air is kept inside the polar vortex with the un depleted air outside the vortex. In spite of ozone is produced primarily at tropical region, due to huge air circulation patterns in the lower stratosphere ozone move toward the poles and concentrate there. In addition to this global motion, strong winter polar vortices are also important for concentrating ozone at the poles. As the air inside the polar vortices becomes extremely cold, then during the dark polar winter there is a necessary condition for polar stratospheric cloud formation. Ozone is destroyed drastically which is created conditionally by Polar stratospheric clouds, providing a surface for chlorine to change into ozone-destroying form. Generally it lasts up to the sun comes up to the spring .In the 1980s, scientists discovered that the ozone layer was thinning in the lower stratosphere, with particularly dramatic ozone loss which is known as the “ozone hole” in the Antarctic spring (September and October). According to different research study it was revealed that thinning in the ozone layer was caused by accumulation of ozone-depleting chemicals such as chlorofluorocarbons or CFCs and to a lesser extent halons [10] (similar compounds with bromine or iodine). In the polar region ice within the clouds attach with CFCs [Fig.2]. In the polar spring the sun comes out again, the ice particles melt, releasing the ozone-depleting molecules from the ice particle surfaces. It is to be noted that chlorine and bromine sources contribute to ozone layer depletion. Research study reveals that chlorine from swimming pools, industrial plants, sea salt, and volcanoes does not reach the stratosphere. In spite of this, ODS are very stable and do not dissolve in rain. Above all no natural processes can remove the ODS from the lower atmosphere.
 Figure 2: Domestic Usage of HFCs
Figure 2: Domestic Usage of HFCs
Climate Change [11] is concerned with how carbon dioxide, methane, and other greenhouse gases are altering the climate system [12]. On the other hand ozone depletion relates the damage of industrially produced chemicals containing chlorine or bromine on the earth’s protective stratospheric ozone layer. Due to greenhouse effect, the solar electromagnetic radiation is absorbed by the Earth, partly radiated outward as infrared energy (heat). Few parts of this infrared energy escapes into space but mostly absorbed by greenhouse gases in the troposphere and finally it radiated back to the Earth as heat energy. The greenhouse effect is a warming of the Earth’s surface that makes it hospitable to life. Without the greenhouse effect, the surface of the Earth would be a frigid -100°F. Greenhouse gases, including water vapor (H2O), carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), are produced as part of the natural system (for example, CO2 and H2O are by-products of respiration and combustion). These greenhouse gases trapped heat letting sunlight through, so it acts the glass roof of a greenhouse. Since the Industrial Revolution, humans have developed technologies (for example, motor vehicles) that produce large quantities of greenhouse gases. The greenhouse effect is enhanced by plenty of all types of man-made or artificial greenhouse gases released into the atmosphere [13]. CO2 plays a significant part for enhancing the greenhouse effect because due to creation of human activities for a long lifetime in the atmosphere. This increase in CO2 comes largely from the burning of fossil fuels (coal, oil, and natural gas) in automobiles and electrical power plants. From the recent news people burning biomass (tropical rainforest burning) to clean which pertains to the problem of too much atmospheric CO2 . This practice is doubly destructive because it also upsets the recycling of carbon. Living organism like green trees act as a major role in taking carbon out of the atmosphere through the mechanism of photosynthesis by which plants use energy from the sun to make food and photosynthesis utilizes carbon from CO2. Naturally, the best source of carbon is green plant. As the presence of phytoplankton in the water, the oceans also are a carbon source which can take up plenty of carbon from atmospheric CO2.
The further accumulation of greenhouse gases commits the planet in irreversible way for further global climatic change. As a consequence ecological, economic, and social disruption affect global climate. Research study reveals that scientists can speculate the effects of global warming may perturb the pattern of ocean circulation, which contribute the overall global climate change. A growing scientific consensus is urging governments and industries to work together despite these uncertainties to reduce man-made greenhouse gases. So the international cooperation regarding damage to the ozone layer happened recently with regard to another problem of atmospheric change.
The combination of gas chromatographs with electron capture (ECD) or mass spectroscopy (MS) detectors, ODS are monitored. Instrumentation can be performed in a monitoring station, with subsequent centralized analysis. Besides, measurements are conducted from various mobile sampling platforms, such as aircrafts, balloons, towers or ships. Remote sensing of CFCs has been reported; Hoffmann et al., 2008; Brown et al., 2011; Kellmann et al., 2012; Minschwaner et al., 2013 [14,15,16,17]. MIPAS instrument on the ENVISAT satellite measuring mid infrared limb radiance spectra, which can be used to retrieve vertical profiles of CFC-11 and CFC-12 [18]. Satellite borne spectroscopic instruments give less accurate measurements than ground based instruments which provide comprehensive spatial coverage [19]. The other important matter is that ODS substitutes are very powerful greenhouse gases like the CFC’s/HCFC’s. The replacement of ODS by these substitutes will therefore lead to a further increase of the greenhouse effect, if they are emitted in huge amounts. Consequently, the greenhouse effect warms up the troposphere and cool down the stratosphere. The stratospheric ozone depletion is exacerbated by cold stratospheric temperature. The substitution of ODS by e.g. fluorinated gases could therefore slow down the recovery of the ozone layer through the climatic effect of these substitutes.
The fertilization of crops can be done by atmospheric CO2, but due to frequent storms and droughts crops will be damaged. The environmental consequences of human activities depends on Human, Places, Environments and different Connections. The two burning topic are global warming and ozone layer depletion, which are continuously paying attention from the media and professionals in the fields of interdisciplinary science. Citizens of the developed countries are paying carbon tax for global warming, which can subsidize energy-efficient technologies or the transfer of such technologies to the developing nations.
There is no conflict of interest.
- “It’s Too Darn Hot,” UCAR Quarterly 27 (1998): 12; National Climate Data Center, Climate of 1999 Annual Review (Washington, DC: National Oceanic and Atmospheric Administration, 2000).
- J. Rye, P. Rubba (2000) “Student Understanding of Global Warming: Implications for STS Education Beyond 2000,” in D. Kumar and K. Chubin, eds., Science, Technology, and Society: A Sourcebook on Research and Practice New York: Plenum 193-230.
- P. A. Newman, J. S. Daniel, D. W. Waugh, E. R. Nash (2007) A new formulation of equivalent effective stratospheric chlorine (EESC). Atmos. Chem. Phys. 7: 4537–4552.
- World Meteorological Organization (WMO) (2014) Assessment for Decision-Makers: Scientific Assessment of Ozone Depletion: 2014, World Meteorological Organization, Global Ozone Research and Monitoring Project—Report No. 56, Geneva, Switzerland, 2014.













