Article / Research Article
1Department of Pure and Applied Chemistry, Kebbi State University of Science and Technology Aliero in Kebbi State, Nigeria
2Department of Chemical Sciences, Federal University of Kashere, P. M. B. 0182, Gombe State, Nigeria
Moshood Hamzat
Department of Pure and Applied Chemistry
Kebbi State University of Science and Technology Aliero
Kebbi State
Nigeria
6 January 2021 ; 21 January 2021
This study showed that kaolinite clay modified with Moringa oleifera pods is a promising low cost adsorbent for the removal of metals from aqueous solution because the resultant composite has higher adsorption capacities, and hence a better metal ions removal efficiency. The efficiencies of these adsorbents for the removal of Pb (II) and Cd (II) ions from aqueous solutions were studied as a function of pH, time, adsorbate concentration and adsorbent dose. Adsorption results showed that pH did significantly affect removal of heavy metal ions between pH 3 and 6. Increasing contact time and initial metal ion concentration increased the sorption capacity of the adsorbent for the metal ions. Adsorbent dosage indicated mainly surface phenomena involving sharing of electrons between the adsorbent surface and the metal ion species. The adsorption of metal ions from aqueous solutions of both metal ions at different initial metal ion concentrations reduced the initial adsorption rates of the adsorption of Pb (II) and Cd (II) by unmodified and modified kaolinite clay.
Keywords/Phrase: Kaolinite clay, adsorption, heavy metals, pH, cadmium, lead.
The search for cheap, efficient and accessible water treatment technologies cannot be overemphasized. Pollutants, especially metallic species introduced into the environment by anthropogenic activities persist indefinitely and eventually accumulate along the food chain through water bodies leading to unprecedented environmental pollution and impacting negatively on the ecosystem [1]. The removal of these metal ions from water and wastewater has become a challenge for researchers [2].
Heavy metals are one of the most worrisome pollutants in the natural environment due to their potential toxicity, persistence and bio-accumulation problems [3]. Direct toxicity to man and other forms of life and indirect toxicity through the food chains are the focus of this concern. In order to combat the threat to the environment and human health, it is highly essential to reduce their concentration levels to a tolerable limit in municipal and industrial effluents prior to their final discharge into the ecosystem. Common sources of heavy metal pollution include discharge from industries such as electroplating, plastics manufacturing, fertilizer producing plants and wastes left after mining and metallurgical processes [4].
Two of the most potentially toxic heavy metal are chromium and lead. Classified as soluble and strongly hydrating cations, these metals are particularly toxic to higher animals, causing kidney and blood diseases among other health disorders. Exposure to lead (Pb) for instance, is widely recognized as a major risk factor for several human diseases, and the structure of ecological systems have made exposure to lead for most people in today’s world formidable [5]. Cadmium which is one of the most important heavy metal used in electroplating and fertilizer industries causes serious toxicological effects at high doses; after uptake it is known to deposit in brain, skin, liver, pancreas and myocardium [6,7].
Over the years, various techniques have been employed to remove metal ions from aqueous solutions, including conventional methods such as ion-exchange, reverse osmosis, electrochemical treatment, evaporative recovery, and adsorption. These techniques have been reported to reduce metal ions to tolerable level in water, but they do not appear to be highly effective due to limitations of removal pH range as well as the high material and operational costs [8].
The quest for a cheaper, effective and eco-friendly method of heavy metal removal from aqueous solution has led to the application low-cost materials as adsorbents for heavy metal ions ion wastewater. Several low cost adsorbents such as biological materials, nanomaterial and clays have been investigated [9]. Among the variety of the natural adsorbents, the composite of kaolinite clay and Moringa oleifera pod is the focus of the study.
Moringa oleifera (drumstick), is a drought tolerant and medicinal tropical tree, available throughout the year. Moringa oleifera seeds are sometimes removed from more mature pods and eaten like peas or roasted like nuts [5]. The flowers are edible when cooked and are said to taste like mushrooms. It has various pharmacological uses as analgesic, antihypertensive and anti-inflammatory effects. The powdered pods of the Moringa oleifera plant has coagulating properties, thus have potential to be used for of water treatment especially turbid water. However, its biosorption behaviour for the removal of toxic metals from water bodies has not been given adequate attention [10].
Kaolinite, (Si)4(Al)4O10(OH)8, is the most common two–sheet layer type of clay [11]. Its formula indicates that there is no substitution of Si4+ with Al3+ in the tetrahedral layer and no substitution of Al3+ with other ions (e.g., Mg2+, Zn2+, Fe2+, Ca2+, Na+ or K+) in the octahedral layer [12]. Thus, the net layer charge of kaolinite is zero. But in nature, kaolinite has a small net negative charge arising on the clay crystals due to protonation/deprotonation influenced by the solution pH. This negative charge, although small, is responsible for the surface not being completely inert; it allows electrostatic interaction with positively charged ions [12,13].
In this study, synergistic combinations of kaolinite clays and Moringa oleifera pods (both being low cost adsorbents) was achieved by combining individual characteristics of each composite to yield a better adsorbent having properties such as high cation exchange capacity, elimination of bleeding, enhanced mechanical strength and higher adsorption efficiency for water treatment. Also, the biomass-modified sorbents was tested for the removal of Pb(II) and Cd(II) ions from aqueous solutions under various conditions; contact time, solution pH, temperature and adsorbent dose, and adsorbate concentration.
The kaolinite clay sample was obtained from a mineral deposit site at Saminaka village, along Koko-Yauri Road, Kebbi State, Nigeria. It was grinded into powdered form, after which it was sieved to 230μm particle size to remove large non-clay fractions from the clay. The Kaolinite clay was then oven dried at 700C and kept in air-tight container for use in the study [14].
House to house collection of the Moringa oleifera pods was adopted using the Basaura Institute Comprehensive vehicle within Birnin Kebbi town, Kebbi State. The sample was washed thoroughly with water and then rinsed with deionized water and oven dried at 1050C for 24 hours, before shielding and grinding in a mortar using pestle. The sample was sieved in a 230μm sieve and kept in air-tight container for use in the study.
Equal weight of Kaolinites clay and Moringa oleifera pod (20g each) were weighed into a 500ml beaker. A 200ml of 0.1M KOH was added to the beaker, followed by stirring. The mixture was transferred into an oven and heated at 1050c for 4 hrs. The mixture was then be allowed to stand for 72hrs and subsequently dried in an oven at 700C. The dried samples mixture was weighed into crucibles and calcined at 3000C in a furnace for 12hrs. The resulting dark powdery material was washed with deionized water to remove all carbon materials on the surface of the composite and was subsequently dried to remove all moisture [15]. The dried sample was stored in an airtight container and labeled as Modified Kaolinite Clay Adsorbent (MKC).
FTIR spectra of adsorbents were obtained using Perkin Elmer infrared spectrometer. For FTIR spectra of the adsorbents, 0.1g of the adsorbents was ground and mixed with 0.5g of KBr salt and pressed into pellets. This pellet was introduced into the Perkin Elmer infrared spectrometer (Nexus 870 FT-IR) which showed peaks corresponding to the spectra of surface functional groups of the adsorbent [4].
Scanning Electron Microscope (JEOL 6400, Japan) was used to obtain microgram scan which showed the surface morphology of the adsorbents.
The synthetic metal ion (Pb2+ and Cd2+) solutions were prepared from AnalaR grade of their salts (Pb(NO3)2 and Cd(NO3)2, from the Kebbi State University of Science and Technology Chemistry Laboratory Aliero, Nigeria.. A stock solution of 1000 mg/L was prepared by dissolving 2.0319g and 1.8287g of Pb2+ and Cd2+ salts respectively in 200cm3distilled water and then made up to mark in a 1L standard volumetric flask. Working solutions of various concentrations were prepared from this stock solution as required.
Replicate batch experiments were used to determine metal adsorption capacity of both the modified and unmodified kaolinite clay samples in 60 mL polyethylene bottles by contacting approximately 1.0 g of the unmodified and modified Kaolinite clay with 20 mL of metal solution, except where otherwise stated, for determining effect of pH, time, and temperature and sorbate concentration. Effect of pH on adsorption of the Pb2+ and Cd2+ was carried out by varying pH from 2±0.2 to 8±0.2 at a contact time of 60 min, effect of time was investigated at a time varied from 10 to 180min, equilibrium experiments were studied at room temperatures of 27±1°C, while varying the concentrations from 100 to 600mg/L at optimum solution pH and contact time. The adsorbent-adsorbate mixtures were shaken on a mechanical shaker during the course of the adsorption experiment and concentrations of metal in the filtrate solutions were determined using the Buck Scientific 205 Atomic Absorption Spectrometer (AAS) with air-acetylene flame on absorbance mode.
The amount of metal ions adsorbed was calculated using the equation:
![]()
Where; Co and Ce are the initial and final metal concentrations in solutions; Qe , V and M are the amount of metal ions adsorbed (mg/g), volume of the solution (mL) used for adsorption and mass (g) of sample, respectively.
The percentage removal of metal ions will be calculated using the following equation;
![]()
Where; R is the removal efficiency of the metal ions adsor¬bent studied; Co is the initial metal ions concentration in solution (mg/L); Ce is the metal ions concentration removed or adsorbed by adsorbent at equilibrium (mg/L).
The infra-red (IR) spectra of both the unmodified kaolinite (KC) and modified kaolinite (MKC) samples were obtained while the band assignments are presented in figures 1a and 1b. The absorption band at 3716 and 3668.3 cm−1 for the unmodi¬fied kaolinite sample represents the inner surface–OH stretching vibration and those at 1021.8 and 918.7 cm−1 represents the Si–O and Al–OH bending vibrations, respectively. An increase in wavelength from 1021.8 cm−1 in the unmodified kaolinite sample to 1035.6 cm−1 respectively, with disappearance of the peak at 918.7 cm−1 in the modified kaolinite sample was ob¬served. The latter peaks were broader and more intense. This suggests that the modification may have been effected at the Si–O− and Al–OH linkages that are negatively charged. The small shift in the 1021.8 cm−1 peak in the unmodified sample to 1035.6 cm−1 in modified sample also confirms the involvement of the Si–O bond linkage in the modification. Similar observation was reported by Adebowale et al. (2006) and Unuabonah et al. (2013). The spectra of the raw clay exhibit a band at 3350 cm−1, which suggests the presence of inner hydroxyl groups (−OH stretching vibration) lying between the tetrahedral and octahedral sheets in the kaolinite clay mineral with a vector orientation near to the (001) plane (pointed in the direction of the vacant octahedral site) [14].
For the modified kaolinite (MKC), the 1035.6 cm−1 Si−O bending vibration shows a significant shift when compared to the raw clay. This suggests that this position is perhaps one of the active sites for interaction of the kaolinite with the Moringa oleifera pods. An amine −N−H band was observed in the com¬posite spectrum at 1606 cm−1 with a characteristic weak −C−N band at 1205 and 1324 cm−1 respectively. The other bands are identical to those of the kaolinite clay. Overall, IR confirms that the MKC material is indeed a composite consisting of ka¬olinite and organic matter arising from the Moringa oleifera pods.
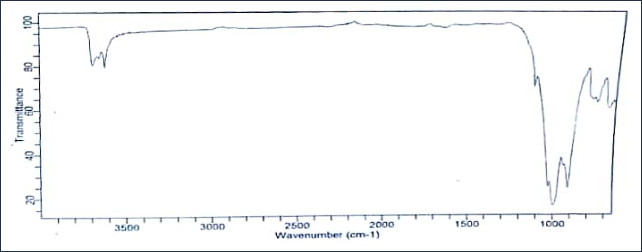
Figure 1a: FTIR spectra of unmodified kaolinite clay
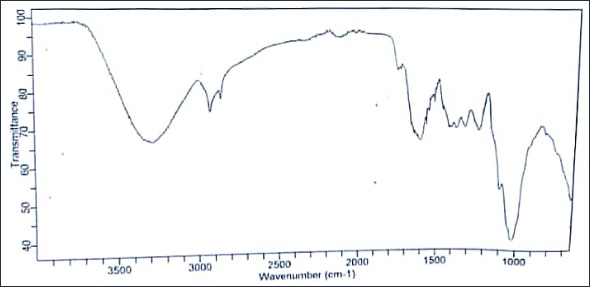
Figure 1b: FTIR spectra of modified kaolinite clay
When viewed under a Scanning Electron Microscope (SEM), the shapes of MKC adsorbent particles were quite similar to those of the unmodified adsorbent (KC). MKC and KC adsor¬bents gave particles with irregular structures. The unmodified adsorbent showed some white particles on the surface of the mineral particles (Figures 2a and 2b). These are likely to be non-clay minerals like sodium, potassium, calcium, iron and magnesium [8]. The SEM images suggest that the MKC ad¬sorbent is a heterogeneous composite (Figure 2b). From the images of MKC adsorbent (Figure 2b), it is observed that the clay particles have reduced sizes.
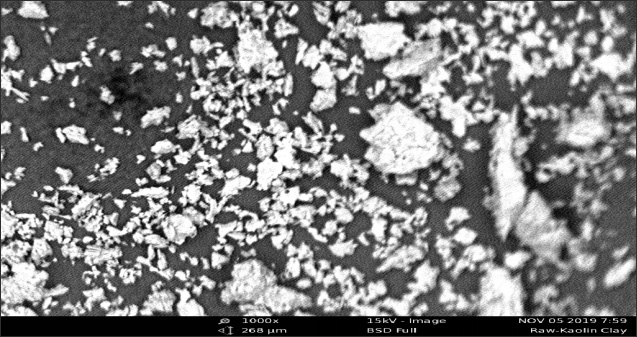
Figure 2a: SEM image of unmodified kaolinite clay

Figure 2b: SEM image of modified kaolinite clay
Figure 3 showed that increasing pH enhanced the uptake of the metal ions, with the modified adsorbent (MKC) showing better performance and Pb ions being more adsorbed onto both adsorbents than Cd ions. This enhanced metal ion uptake by kaolinite was also reported by Adebowale et al. (2006) and Unuabonah et al. (2013). This may be as a result of increased overall negative charge on both the unmodified and modified kaolinite samples especially between pH 4 and 6. In addition, increasing pH decreases the concentration of H+ therefore reducing the competition between metal ions and protons for adsorption sites on the particle surface. Another factor that could also contribute to enhancing metal ion adsorption is the increasing pH which encourages metal ion precipitation from the solution in the form of hydroxides.
Several reasons may be attributed to the increased adsorption of metal ions by unmodified adsorbent relative to adsorbate solution pH. The surface of the kaolinite clay sample contains a large number of active sites and may become positively charged at very low pH, thus increasing the competition between H+ and the metal ions for available adsorption sites. However, as pH increases, this competition decreases as these surface active sites become more negatively charged, which enhances the adsorption of the positively charged metal ions through electrostatic force of attraction [12].
These results showed that pure and modified kaolinite clays could be used for adsorption of metals in solutions having pH between 3 and 6. This is a wide pH range of adsorption unlike those of most adsorbents in literature with narrow optimum pH range of adsorption; hence, these adsorbents may be applied for pollutants adsorption from a wide range of aqueous acidic solutions.
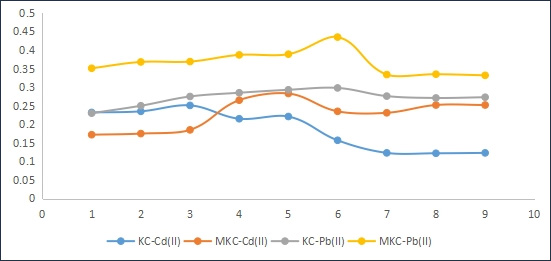
Figure 3: Effect of pH on adsorption of Pb (II) and Cd(II) onto KC and MKC adsorbents
From figure 4, it was observed that modification of kaolinite clay enhanced adsorption of Pb (II) and Cd(II) ions ions as well as altered the rate of adsorption these metal ions by the adsorbent. Unlike the unmodified adsorbents, there was a sharp increase in the adsorption of both metal ions on the modified adsorbent within the first 30 min of the reaction. For adsorption of lead on modified adsorbent, equilibrium was reached after 60 min with 96.8% adsorption while for the unmodified adsorbent, equilibrium was attained after 60 min with 87.5% adsorption. However, for cadmium adsorption, equilibrium condition was attained after 60 min for the modified adsorbent and 30 min for the unmodified adsorbent with 78.6 and 68.9% adsorption, respectively. This accelerated adsorption of these metal ions onto modified kaolinite clay may be as a result of the blocking of some pores on the surface of unmodified kaolinite clay [16]. As a result, Pb and Cd ions may not be able to diffuse further into the pores and this will increase the overall rate of adsorption. A similar observation was reported by Sanusi et al. (2016).
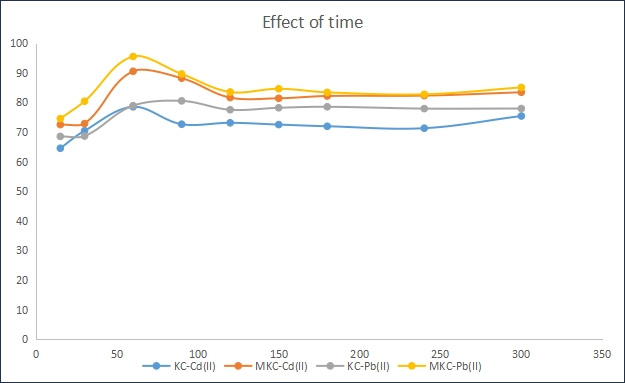
Figure 4: Effect of contact time on adsorption of Pb (II) and Cd(II) onto KC and MKC adsorbents
Figure 5 shows the effect of adsorbent dose on the adsorption capacity of the KC and MKC. Increase in the dosage of both adsorbents from a range of 0.5–3.0 g resulted in a decrease in equilibrium adsorption capacity, qe, of the adsorbents. However it was observed that the percentage of adsorbate adsorbed increased with increase in adsorbent dose until an equilibrium is reached at a mass of 2g for both Pb (95.5%) and Cd (97.8%). A similar trend has been reported in a previous study by Unuabonah et al. (2013). The increased percentage adsorption of these metal ions with increasing adsorbent dose is possibly due to increased surface negative charge and decrease in the electrostatic potential near the solid surface that favors sorbent– solute interaction [17]. However, the decreased equilibrium adsorption capacity, qe, of the modified clay sample for the metal ions, as its dosages were increased, can be attributed to decreasing total surface area of the adsorbent and an increase in diffusion path length, which is the result of aggregation of adsorbent particles [14].
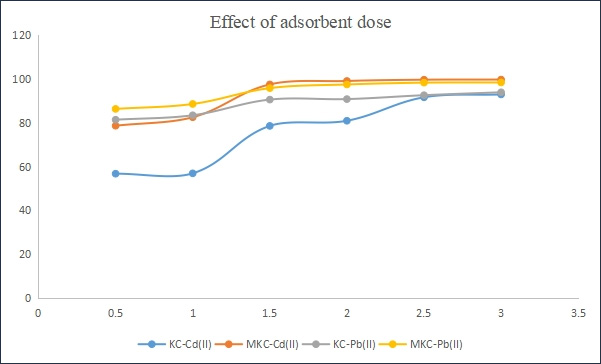
Figure 5: Effect of adsorbent dose on adsorption of Pb (II) and Cd(II) onto KC and MKC adsorbents
It was observed from figure 6 that increasing initial metal ion concentration from 100 to 600 mg/L resulted in an increase in the metal ions adsorbed for both modified and unmodified kaolinite adsorbent until adsorption equilibrium is reached at 300mg/L. At very low initial concentrations 83.7% of Pb ions were adsorbed on modified kaolinite clay compared with 78.5% of Pb ions on the unmodified sorbent. However, 58.1% Cd ions were adsorbed on modified kaolinite clay whereas 31.5% of Cd ions were adsorbed on unmodified kaolinite clay samples. With increasing metal ion concentration, there is increase in the amount of metal ion adsorbed due to increasing driving force of the metal ions towards the active sites on both the modified (83, 91.6%) and unmodified (62.7, 78.9%) ad-sorbents for Cd and Pb ions respectively. The sorption of both metals is more favorable on the modified kaolinite adsorbent than on the unmodified kaolinite adsorbent due to enhanced adsorption capacity qe of the MKC. The results obtained were in correlation with that reported by Stephen et al. (2017) [18].
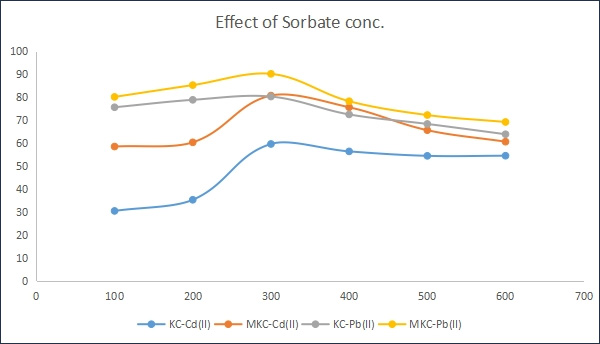
Figure 6: Effect of adsorbate concentration on adsorption of Pb (II) and Cd(II) onto KC and MKC adsorbents
The modification of kaolinite clay mineral was found to be on both silanol (Si–O) and aluminol (Al–OH) sites of the clay mineral and not on the edge – hydroxyl groups on the surface of the adsorbent. Adsorption studies on the aqueous solutions of both Pb (II) and Cd(II) ions revealed that adsorption of both metal ions is suppressed by the presence of either metal ion concentration with respect to effect of time, solution pH and adsorbent dosage. There was an observed increase in the adsorption capacity of the kaolinite after modification with Moringa oleifera pods which corresponds to its increase in cation exchange capacity. The results showed that the modified kaolinite adsorbent has the potential of holding heavy metal ions from the aqueous solution. The low cost composite adsorbent MKC, has a strong potential for replacing other expensive methods in treatment of water laden with heavy metals.
- Adelaja OA, Amoo IA, Aderibigbe AD (2011) Biosorption of Lead (II) ions from aqueous solution using Moringa oleifera pods. Archives of Applied Science Research 3: 50-60.
- Abdul DAS (2007) Economic Importance of Moringa oleifera in Tafa Local Government Area of Niger State. NDE Project. Federal College of Forestry Mechanization, Kaduna, Nigeria.
- Abelkop AD, Graham JD, Royer TV (2016) Persistent, Bioaccumulative, and Toxic (PBT) Chemicals. Technical Aspects, Policies, and Practices. CRC Press.
- Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of Toxic Metals from Aqueous Solutions by Bacteria Strains Isolated from Metal Polluted Soils. Process Biochemistry 39: 909-916.
- Dietrich KN, Douglas RM, Succop PA, Berger OG, Bornschein RL (2001) Early Exposure to Lead and Juvenile Delinquency. Neurotoxicology and Teratology 23: 511- 518.
- Goyer RA, Clarkson TW (1996) Toxic Effects of Metals. The Basic Science of Poisons, McGraw-Hill Health Professions Division. Fifth Edition. ISBN: 71054766.
- Abe R, Ohtani K (2013) An Ethnobotanical Study of Medicinal Plants and Traditional Therapies on Batan Island, the Philippines. Journal of Ethnopharmacology 145: 554- 565.
- Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2006) The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. Journal of Hazardous material 134: 130-139.
- Olu-Owolabi BI, Alimoh H Alabi, Emmanuel I Unuabonah, Paul N Diagboya (2016) Calcined bentonite-biomass composites for removal of aqueous metal ions. Journal of chemical and engineering technology 4: 1379-1383.
- Patrick L (2006) Lead Toxicity, a review of the literature. Part I: Exposure, Evaluation, and Treatment. Alternative Medicine Review 11: 123-128.
- Connell DW, Birkinshaw C, Dwyer TF (2008) Heavy Metal Adsorbents Prepared from the Modification of Cellulose: A Review. Journal of Bioresource Technology 9: 6709-6724.
- Jamo HU, Abdu SG (2014) Structural Analysis and Surface Morphology of Kaolin. Science World Journal 9: 33- 37.
- Xiaoyan Z, Zhichao Z, Xinrong L, Chunjie Y (2016) Defects in structure as the sources of the surface charges of kaolinite, Applied Clay Science 3: 124-136.
- Sanusi KA, Babayo AU, Isyaka MS (2016) Evaluation of the Application of Carica papaya Seed Modified Feldspar Clay for Adsorption of Pb+2 and Cu+2 in Aqueous Media: Equilibrium and Thermodynamic studies. Journal of Environmental & Analytical Toxicology 6: 02-09.
- Unuabonah IE, Gunter C, Weber J, Lubahn S, Taubart A (2013) Hybrid Clay: A New Highly Efficient Adsorbent for Water Treatment. Journal of Industrial and Engineering Chemistry 132: 252-255.
- Dąbrowski A (2001) Adsorption from Theory to Practice. Advances in Colloid and Interface Science 9: 135-224.
- Stephen N, Edmore S, Netai MM, Munyaradzi S (2017) Comparative Biosorption of Pb2+ and Cd2+ ions from Aqueous Solution Using Moringa oleifera Plant Parts: Equilibrium, Kinetics and Thermodynamic Studies. African Journal of Biotechnology 16: 2215-2231.
- Da Silva JPV, Serra TM, Gossmann M, Wolf CR, Meneghetti MR, Meneghetti SMP (2010) Moringa oleifera oil: Studies of Characterization and Biodiesel Production. Biomass and Bioenergy 34: 1527-1530.













