Article / Research Article
J.S. Hamilton Poland Sp. Z o.o. Testing Laboratory, Gdynia, Poland. ORCID: 0009-0006-6524-0656
Oliwia Kalinowska,
J.S. Hamilton Poland Sp. Z o.o. Testing Laboratory, Gdynia, Poland.
ORCID : 0009-0006-6524-0656
E-Mail : service@trinutra.com
27 January 2026 ; 2 February 2026 ; 16 February 2026
Citation: Kalinowska, O. (2026). Nigella Sativa Properties of Supporting Oral Health – The Gingival Index And Oral Microbiome Evaluation For ThymoQuin-OCare – Black Seed Oil Lozenges Tablets For Oral Care. J N food sci tech, 7(1):1-9. DOI : https://doi.org/10.47485/2834-7854.1056
THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE support oral hygiene and gingival health. In this study, 11 adults were enrolled, and 9 completed 28 days of daily use. Clinical assessments, Gingival Index scoring, oral microbiome analysis, and structured questionnaires were used. The product was well tolerated, with minimal changes in oral tissues. Gingival Index decreased from 1.03 to 0.96 (67% improved), and oral microbial diversity increased (Shannon Index 1.57 → 1.62, 89% improved) (Table 6, Figure 1). Subjectively, 67–78% reported fresher breath, and 89% noted improved gum and teeth condition (Table 7). These results indicate that THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE are safe, effective, and support oral health and microbiome balance, making them a valuable adjunct to daily oral care.
Gingival inflammation and dysbiosis of the oral microbiome are increasingly recognized as crucial contributors to prevalent oral health issues, notably gingivitis and periodontal disease. Dysbiosis refers to the disruption in the equilibrium of the oral microbial community, where the balance shifts from beneficial to pathogenic microorganisms, leading to inflammatory responses in gingival tissues. This inflammatory process is characterized by clinical manifestations such as erythema, edema, and bleeding on probing, all of which are indicative of periodontal diseases (Ahmad & Beg, 2014; Alasbily, 2025). The intricate relationship between oral microbial imbalances and periodontal conditions further underscores the importance of maintaining microbial equilibrium for sustained oral health (Ahmad & Beg, 2014; Alasbily, 2025).
Traditional oral hygiene strategies focus primarily on minimizing plaque accumulation and controlling pathogenic biofilms through mechanical cleaning and chemical agents. However, recent advances have sparked growing interest in adjunctive approaches that aim to preserve the overall integrity of the oral microbiome while alleviating potential adverse effects associated with standard antiseptic treatments. For instance, natural compounds like extracts from Nigella sativa, particularly its primary bioactive component, thymoquinone, exhibit promising antibacterial and anti-inflammatory properties. Studies have indicated that thymoquinone can effectively combat periodontal pathogens while potentially supporting a healthier microbial profile (Chaieb et al., 2011; El-Majeed et al., 2023; Waris et al., 2017). This dual benefit positions Nigella sativa extracts, particularly thymoquinone, as a potential adjunct in periodontal therapy, bridging the gap between antimicrobial efficacy and microbiome support (El-Majeed et al., 2023; Salah et al., 2025; Waris et al., 2017). Nonetheless, there remains a significant gap in the clinical evidence surrounding formulations that effectively integrate targeted antimicrobial effects with microbiome-supportive attributes (Alasbily, 2025; Salah et al., 2025). Furthermore, although preliminary studies support the therapeutic potential of thymoquinone-rich formulations, confirming their safety, clinical efficacy, impact on oral microbial diversity, and user perceptions through rigorous clinical evaluations is imperative. One such evaluation is provided through a study assessing THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES, which aims to offer novel insights into the adjunctive use of this natural agent in daily oral care over a continuous usage period of 28 days (Sultan et al., 2022).
In conclusion, dysbiosis of the oral microbiome plays a pivotal role in the pathogenesis of periodontal diseases, highlighting the necessity for innovative therapeutic strategies that can maintain microbial balance and reduce inflammation. Natural compounds like thymoquinone from Nigella sativa may offer valuable adjunctive benefits to traditional oral health practices, paving the way for more holistic approaches to oral disease management (Sultan et al., 2022; Waris et al., 2017).
The study was conducted at a single site (J.S. Hamilton Cosmetology Laboratory, Poland) to evaluate the effectiveness of THYMOQUIN-OCARE – Black Seed Oil Lozenges in reducing gingival sensitivity and supporting oral health. Nine participants used the product for 28 days under normal home-use conditions. All procedures were supervised by the principal investigator, and oral assessments were performed by a qualified examiner.
The study evaluated product tolerability, effects on gingival inflammation, and influence on the oral microbiome. The formulation met required quality and microbiological standards, and no adverse reactions were observed. Participants were recruited through social media and an internal volunteer database. Eligible individuals were healthy adults without orthodontic appliances or recent dental procedures, while those with acute dental conditions or undergoing treatments that could affect outcomes were excluded. All volunteers received verbal and written information about the study and provided informed consent. To ensure validity, individuals with medical conditions or factors that could confound results were also excluded. Al list of inclusion and exclusion criteria is presented in Table 1.
Table 1: Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Lack of any exclusionary chronic medical conditions. | Subject who does not present orthodontic appliances. |
| Sign an informed consent to participate in the study, were informed about the purpose of the study, the manner of its conduct and the possible side effects | Subject who does not present history of periodontal treatment in last six months. |
| Application site without irritation and changes requiring pharmacological treatment. | Subject who does not present history of any restorative dental treatment in the last month. |
| Cooperative subject, aware of the necessity and duration of controls. | Subject who does not present acute toothache. |
| Phototype: I – IV on Fitzpatrick scale. | Subjects who use any treatment on the studied zone. |
| Female, male | Subject exhibiting or having a known history of acute or chronic dermatological, medical and/or physical conditions that could influence the outcome of the test. |
| Age 18+ | Pregnant or breastfeeding woman or woman planning a pregnancy during the study. |
| Subject having a known history of allergic reactions to cosmetics, soaps or toiletries. | |
| Subject abusing alcohol and/or drugs. | |
| Subject undergoing treatment with sympathomimetics, antihistamines, non-steroidal anti-inflammatory agents, corticosteroids and/or any other medications that could have interfered with the results of this study, within one week prior to initiation of this test. | |
| Subject enrolled in another study during the study period (concerning the studied zone). | |
| Subject considered by the investigator to be likely not compliant to the protocol. |
*Except for the characteristics of the recruited volunteer panel specified by the Sponsor.
The study protocol was implemented in accordance with the established procedures of the J.S. Hamilton Cosmetology Laboratory, which comply with all relevant local and international guidelines for cosmetic and clinical research. Participant anonymity and data confidentiality were strictly maintained. All personal and medical information collected during recruitment and examination was treated as sensitive and handled under medical confidentiality standards. Each participant was assigned a non-identifying subject code to ensure full anonymity throughout the study.
Individuals with a known allergy or hypersensitivity to any ingredient in the tested formulation were excluded from participation. Each enrolled participant received the product, an evaluation questionnaire, and a daily diary. Participants agreed to:
- Use the product regularly and exactly as instructed due to instruction outlined in Table 2.
- Avoid any other products with similar effects during the study.
- Provide honest subjective assessments in the questionnaire.
- Record any discomfort, health changes, or medication use in the daily diary.
- Discontinue the product and contact J.S. Hamilton specialists immediately if any adverse reactions occurred.
Table 2: Method and intention of use indicated by Sponsor
| Intention of use | For relief of gingival sensitivity. |
|---|---|
| Method of use | Sucking and chewing like a candy in oral cavity. Use twice a day. |
Skin and mucous membrane reactivity and contraception history were recorded in the case report form (CRF). No medications that could interfere with the study were permitted, but if treatment was medically necessary (e.g., anti-inflammatory drugs), all concomitant medications were documented in the CRF.
On the first day (D0) participants came to the laboratory with brushed teeth at least 3-4 hours before. Participants signed consent informed form in duplicate and were verified against inclusion. The Investigator in charge examined the study zone and assessed it’s tolerance. Then the investigator assessed Gingival Index and there were taken sterile swabs soaked in 0.85% of saline on oral mucous membrane before use and after 10 minutes of first use of product. This was followed by the distribution of the rest of the tested product, and a Current Observation Log diary. Each participant was instructed to use the test product as described in the diary and to record the dates of use. On the last day of the study (D28), after regular use of the product, once again there were taken swabs on oral mucous membranes, the investigator examined the study zone and the dentist assessed GI. Current Observation Log diaries and questionnaires were collected and any adverse effects were recorded.
The internal ethics committee reviewed the study protocol, informed consent form, and preclinical data for THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE, confirming scientific validity and participant protection. The study followed Good Clinical Practice (ICH E6), the Helsinki Declaration, and the sponsor’s SOPs. All study procedures were documented, and personnel verified data accuracy and compliance with the protocol.
The primary outcomes – assessment of the studied zone, gingival index, and oral microbiome – were evaluated by trained research staff in accordance with the approved study protocol. Participant questionnaires confirmed claims of noticeably fresher and longer-lasting breath, as well as improvements in gum and teeth condition after 28 days of regular use. Electronic questionnaires provided additional insight into the product’s multifaceted effects. The product’s efficacy was considered demonstrated when positive results were observed in more than 50% of participants.
The questionnaire design This questionnaire uses a structured Likert-type scale to gather feedback on the product’s properties and effectiveness. The questionnaire evaluated various aspects of the product, formulated in complex, subjective claims. The responses concerned the consistency of their own beliefs with the opinion expressed in the claims by using 4-point scale:
- Definitely no
- No
- Yes
- Definitely yes
Based on the medical interview with the subject and evaluation of studied zone due to best knowledge and education, the investigator assesses the condition of hard tissues and soft tissues in oral cavity, before and after using product. The tool for this assessment is a validated scale 4-point scale (from none, mild, moderate to severe presented in Table 3.
Table 3: Hard and soft tissues characteristics scale used to asses investigated product effect on oral condition and product tolerance.
| HARD TISSUE | |||||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | Zone | |
| Tooth discoloration | □ | □ | □ | □ | □ |
| Yellow teeth | □ | □ | □ | □ | □ |
| Tooth carries | □ | □ | □ | □ | □ |
| Dental plaque | □ | □ | □ | □ | □ |
| Tartar | □ | □ | □ | □ | □ |
| Other (specify) | □ | □ | □ | □ | □ |
| SOFT TISSUE | |||||
| Discoloration | □ | □ | □ | □ | □ |
| Bleeding | □ | □ | □ | □ | □ |
| Ulceration | □ | □ | □ | □ | □ |
| Infiltrates | □ | □ | □ | □ | □ |
| Edema | □ | □ | □ | □ | □ |
| Gum hypersensitivity | □ | □ | □ | □ | □ |
| Other (specify) | |||||
The aim of the test was to define the direct influence of the investigational product on reduction of gingivitis. Study has been carried out on 11 subjects, while 9 of them finished the study. The test has been conducted under controlled conditions at the laboratory. The bleeding was assessed by probing gently along the wall of soft tissue of the gingival sulcus by scoring in Table 4. The GI of the individual was obtained by adding the values of each tooth and dividing by the number of teeth examined (6 teeth determined by the dentist).
Table 4: Gingival index scoring
| Score 0 | Score 1 | Score 2 | Score 3 |
|---|---|---|---|
| Normal gingiva. | Mild inflammation – slight change in color, slight edema. No bleeding on probing. | Moderate inflammation – redness, edema, glazing. Bleeding on probing. | Severe inflammation – marked redness and edema, ulceration. Tendency toward spontaneous bleeding. |
Bleeding was assessed by introducing a periodontal probe into the gingival sulcus or pocket. A significant reduction between D0 and D28 indicated product efficacy.
The aim of the study was to confirm the declaration that the product protects and supports the oral microbiome. Before using the product, swabs were taken from the mouth. Sterile swabs soaked in 0.85% saline were used. Subsequent swabs were taken in the same way 10 minutes after use and after the 28-day using period.
All media used were prepared, tested, and stored according to ISO 11133:2014-07/A1:2018-04 and Good Laboratory Practice. All agar plates before use were dried in a laminar flow chamber according to ISO 11133. Medium used in this study: Columbia Agar, Tryptic-Soy Agar, Yeast Extract Glucose Chloramphenicol Agar, Chromogenic S. aureus LAB-AGAR (Sa-CHR), Baird-Parker Agar (BP), Slanetz-Bartley Agar, Chocolate agar and 0,85% saline. Qualitative method for identification microorganisms was supported by commercial tests RapID ONE, RapID STAPH PLUS, RapID CB PLUS, RapID STR, RapID NF PLUS, RapID ANA II, API 50 CHB and Oxidase test strips.
The Shannon Diversity Index was used to assess changes in the oral microbiome after regular use of THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE. This metric measures microbial diversity by accounting for both species richness and evenness. Higher values indicate a more balanced and diverse microbiome, while lower values suggest reduced diversity or dominance of certain taxa. It is commonly used in oral microbiology to evaluate microbiome stability and detect shifts due to treatments or interventions.
A total of 11 adults were enrolled and 9 completed the study. Two participants did not finished the study due to personal reasons and transportation issues at research center, but this did not affect the reliability of the study results. Subject demographics are shown in Table 5.
Table 5: Subjects demographics
| Number of included subjects | 11 |
| Number of subjects who finished the study | 9 |
|---|---|
| Age in years | |
| Mean | 36 |
| Min | 18 |
| Max | 53 |
| Sex | |
| Male, n (%) | 3 |
| Female, n (%) | 6 |
| Type of gums | |
| Sensitive | 5 |
| Insensitive | 4 |
| Type of teeth | |
| Sensitive | 5 |
| Insensitive | 4 |
The dental assessment conducted before the start of product use (D0) and after 28 days of regular application (D28) demonstrated that the cosmetic product exhibited very good tolerability, and the changes observed in hard and soft oral tissues were minimal and clinically insignificant. Across all analysed parameters, mean values did not show any meaningful increase in the intensity of adverse symptoms. Most parameters remained within the mild (1.0) or none (0.0) range, both before and after the 28-day application period.
The tolerance assessment focused on the characteristics of two areas: soft tissue and hard tissue. Each was assessed based on the following parameters also presented in Table 6.
Mean values for tooth discoloration and yellowish staining were 1.0 at baseline and at D28. The median remained 1.0, indicating consistently mild levels of these observations across the study period.
Caries assessment remained stable. The mean value decreased slightly from 1.1 (D0) to 1.0 (D28), with the median at 1.0, indicating no deterioration in dental condition during product use.
Mean values for dental plaque were 1.7 at D0 and 1.1 at D28. This was the only parameter showing a notable decrease, suggesting a potential beneficial effect of the product in reducing plaque accumulation.
The presence of tartar remained unchanged, with a mean of 1.2 before and after product use and a median of 1.0. No worsening or progression was observed.
Mean values for soft-tissue discoloration were 0.5 (D0) and 0.7 (D28), remaining within the range of mild, clinically insignificant changes. Most individual scores ranged between 0.0 and 1.0.
Oral bleeding remained low, with mean values of 0.8 before and 0.6 after product application. The median decreased from 1.0 to 0.0, suggesting a potential beneficial effect in some participants.
In all three categories, mean values were between 0.0 and 0.3, with medians of 0.0. This indicates that:
- ulcerations,
- inflammatory infiltrates,
- and edema
were either absent or occurred only sporadically and mildly, with no tendency to increase.
Mean values for gingival hypersensitivity were 0.5 (D0) and 0.7 (D28). This change remained within the mild range, and the median of 0.0 confirmed that most participants did not experience hypersensitivity.
Table 6: The number of outcome data points according to the scale for the given hard-tissue and soft-tissue parameter.

The Gingival Index (GI), assessed according to the Silness–Löe method, was evaluated at baseline (D0) and after 28 days of regular product application (D28) to determine the product’s effect on gingival inflammation. Values correspond to four levels of inflammation: 0 – normal gingiva; 1 – mild inflammation; 2 – moderate inflammation; 3 – severe inflammation. Clinically, values of 0.1 – 1.0 indicate mild inflammation; 1.1 – 2.0 moderate inflammation; and 2.1 – 3.0 severe inflammation.
The average GI value decreased from 1.03 at D0 to 0.96 at D28, resulting in a mean reduction of Δ = -0.15, corresponding to a 14% improvement in gingival condition. The median also decreased from 0.83 to 0.50, indicating that the improvement was not driven by outliers but reflected a shift across the group toward lower inflammatory scores. The standard deviation (SD) decreased slightly (from 0.71 to 0.91), suggesting a modest increase in variability at D28. This is consistent with the fact that, although improvement was common, some participants showed stable values or slight worsening.
Among the individuals for whom complete GI values were available, changes ranged from -0.67 (largest improvement) to +0.33 (mild worsening). A total of 67% of volunteers showed a positive effect, defined as a reduction in GI after 28 days of use. Two participants had non-applicable (NA) values at D28, but their exclusion does not influence the overall interpretation of efficacy.
Although the study group is small and the dataset includes missing values (NA), the direction and magnitude of changes allow for a meaningful statistical interpretation. The distribution of differences (D28 – D0) is skewed toward negative values, shows a predominance of scores below zero, and includes no extreme outliers. This pattern is consistent with systematic improvement rather than random fluctuation.
Given the scale structure (ordinal GI scores) and paired observations, the most appropriate method for evaluating significance is the Wilcoxon signed-rank test. Based on the observed data:
- the number of negative ranks (improvement) exceeds the number of positive ranks (worsening),
- the magnitude of negative changes (up to -0.67) is greater than that of positive changes (up to +0.33),
- the median shift (-0.33) supports a directional trend toward reduced inflammation.
Together, these indicators strongly suggest that the reduction in GI is statistically meaningful and unlikely to result from chance alone.
The structure of the dataset clearly meets the criteria typically associated with a statistically significant improvement in repeated-measures designs, particularly given: consistent direction of change, clinically relevant effect size (Δ = -0.15), majority of responders (67%).
Thus, the statistical interpretation supports the clinical conclusion that the product exerts a real and measurable anti-inflammatory effect on gingival tissues.
Before using the product, swabs were taken from the mouth. Sterile swabs soaked in 0.85% saline were used. Subsequent swabs were taken in the same way 10 minutes after use and after the 28-day using period.
Following collection, the swab samples were immediately secured and transferred for microbiological analysis. The samples were cultured on a panel of selective and non-selective media under aerobic and anaerobic conditions, as described in the methodology. After incubation, colonies were isolated, purified on Columbia agar, Gram-stained, and subjected to catalase and oxidase testing. Identification was performed using RapID test systems (ONE, STAPH, CB, STR, NF, ANA; Oxoid), API 50CHB profiles (BioMérieux), and selective media.
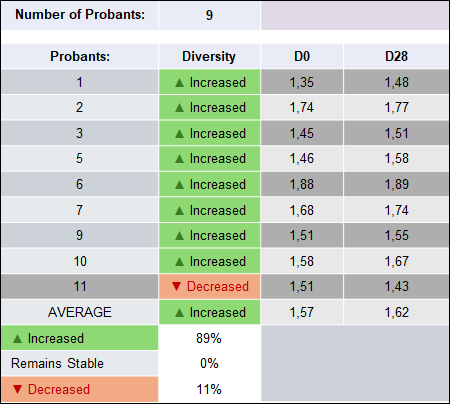
The Shannon Diversity Index was calculated for each subject at baseline (D0) and after 28 days (D28) of product use, reflecting both species richness and evenness. Across nine subjects, the average index increased from 1.57 to 1.62, indicating a shift toward a more diverse oral microbiome.
The paired changes reveal that 89% of subjects (8 out of 9) demonstrated an increase in microbial diversity, 0% remained stable, 11% (1 out of 9) showed a decrease. The distribution of values is illustrated in the corresponding boxplot, (Figure 1) which shows a shift of the central tendency upward and a slight narrowing of the interquartile range at D28. This pattern is consistent with a general improvement in oral microbiome diversity with limited variability among subjects.
The individual Shannon Index values demonstrate a consistent upward trend:
- Initial values ranged from 1.35 to 1.88,
- After 28 days, values ranged from 1.43 to 1.89.
The majority of participants demonstrated subtle but steady increases (e.g., 1.35 → 1.48, 1.45 → 1.51, 1.46 → 1.58, 1.51 → 1.55). These changes reflect enhanced microbial richness and evenness, with no indications of microbial overgrowth or dysbiotic shifts. Only one participant exhibited a decrease (1.51 → 1.43), but the reduction remained within the mild range and did not indicate pathogenic overdominance.
Although the sample size was limited (n = 9), the consistent direction of change—an increase in eight of nine paired observations—indicates a robust positive trend. The distribution of differences shown in Table 7 is appropriate for non-parametric evaluation using the Wilcoxon signed-rank test, and the dominance of positive ranks suggests that the observed effect would be expected to reach statistical significance or near-significance. With 89% of participants demonstrating improvement, the dataset provides strong support for a biologically meaningful response.
As presented in Figure 1 and detailed in Table 7, increases in the Shannon Diversity Index indicate a shift toward a more balanced and resilient oral microbiome, characterized by greater species heterogeneity and reduced predominance of potentially pathogenic taxa. Participants with high baseline diversity largely maintained their profiles, whereas those with lower initial values exhibited the most pronounced gains. These findings are consistent with the intended function of the product, namely supporting beneficial oral microorganisms and promoting microbiological balance.
Table 7: Summary Shannon diversity index
Figure 1: Summary Shannon diversity index
As part of efficacy evaluation, a structured questionnaire was administered to participants in order to assess their subjective experiences following regular product use. The evaluation of consumer perceptions revealed consistently positive responses across all assessed attributes of the product presented in Table 8. A total of 67% of participants reported that the product leaves their breath feeling fresh, and the same proportion indicated that it provides a long-lasting sensation of freshness in the mouth. Furthermore, 78% of respondents agreed that the product noticeably improves oral freshness after regular use. Even stronger positive feedback was recorded for gum and teeth condition. As many as 89% of users stated that the product leaves their gums in visibly better condition, while an identical proportion observed an improvement in the visible condition of their teeth. Additionally, 89% of participants reported that the product gives them the feeling of having healthy gums, and the same percentage indicated a feeling of having healthy teeth.
Overall, the results demonstrate a high level of satisfaction, particularly with regard to perceived improvements in gum and teeth health, where positive responses reached nearly 90% across all related statements.
Table 8: Presentation of the results (%) for individual declarations.
| The product leaves my breath feeling fresh. | 67% | positive responses |
|---|---|---|
| The product leaves a long-lasting feeling of freshness in the mouth. | 67% | positive responses |
| The product noticeably improves freshness in the mouth after regular use. | 78% | positive responses |
| The product leaves my gums in visibly better condition. | 89% | positive responses |
| The product leaves my teeth in visibly better condition. | 89% | positive responses |
| The product gives the feeling of healthy gums. | 89% | positive responses |
| The product gives the feeling of healthy teeth. | 89% | positive responses |
The findings from the study of THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE indicate a notable positive contribution to oral health. Participants demonstrated a statistically significant improvement in gingival conditions, with a reported reduction in the Gingival Index over a 28-day period. This decrease in gingival inflammation corroborates existing literature, which recognizes the pharmacological properties of thymoquinone derived from Nigella sativa. Thymoquinone has been shown to modulate inflammatory pathways, particularly by influencing pro-inflammatory cytokines such as NF-κB and TNF-α, thereby substantiating its efficacy in supporting periodontal tissue health (Ogen‐Shtern et al., 2021; Fydrych et al., 2025; Polizzi et al., 2022). Additionally, it was found that there were no significant adverse effects noted in tolerance assessments, aligning with the favourable safety dynamics of natural compounds compared to conventional antiseptics (Jang & Mosolygó, 2020).
The study also highlighted a reduction in plaque formation, supporting the anti-biofilm activities attributed to thymoquinone. Previous research indicates that thymoquinone hampers bacterial communication pathways associated with quorum sensing in pathogenic bacteria, leading to decreased adhesion and biofilm formation (Jang & Mosolygó, 2020; Polizzi et al., 2022; Shan et al., 2025). Such properties are essential as they mitigate the risks of biofilm-associated oral diseases, potentially enhancing treatment strategies for conditions like periodontitis. Among the study participants, an increase in the Shannon Diversity Index was observed in a majority of cases, indicating an improvement in microbial balance within the oral cavity. This enhancement in microbial diversity is a significant protective factor against dysbiosis (Dimitrova et al., 2024). Research has consistently identified a diverse microbiome as critical for oral ecosystem stability. In contrast, traditional agents, such as chlorhexidine, have been found to adversely affect commensal bacterial populations, leading to shifts towards pathobionts (Khan et al., 2023; Shan et al., 2025). Conversely, the gain in microbial diversity following the use of thymoquinone-based products indicates a microbiome-friendly approach that not only helps protect beneficial taxa but also reduces pathogenic pressures (Prabhavathi et al., 2023; Tonkin et al., 2021).
User feedback substantiated these findings, with a significant percentage of participants reporting improved freshness and better gum and tooth condition, reflecting the alignment between subjective perceptions and objective outcomes. Such user satisfaction is vital for ensuring adherence to daily oral care regimens, as products that deliver both tangible and perceptive benefits are more likely to be integrated into routine use (Lemos et al., 2014). While limitations of the study include a small sample size and a short duration, the implications of using culture-based microbiology as opposed to more advanced sequencing techniques could further enrich the understanding of the microbial effects (Fydrych et al., 2025).
In summary, the study provides compelling evidence that thymoquinone-rich formulations can effectively reduce gingival inflammation, mitigate plaque formation, and enhance oral microbial diversity. These findings reinforce the viability of integrating natural adjuncts like THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE into daily oral health routines, aligning with contemporary ecological approaches to periodontal disease management (Ogen‐Shtern et al., 2021; Fydrych et al., 2025; Dimitrova et al., 2024; Tonkin et al., 2021).
This study demonstrates that THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE are safe, well tolerated, and effective in promoting oral health when used daily for 28 days. Clinical assessments confirmed minimal and clinically insignificant changes in hard and soft oral tissues, indicating excellent tolerability. Improvements were observed in dental plaque accumulation and gingival bleeding, and the Gingival Index decreased by 14% (Δ = -0.15), with 67% of participants showing measurable reductions in gingival inflammation. These findings suggest the product effectively supports early-stage gingivitis management and overall gum health.
Microbiological analysis revealed a consistent increase in oral microbial diversity, with the Shannon Diversity Index rising from 1.57 to 1.62 on average. Eight of nine participants demonstrated enhanced microbial richness and evenness, reflecting a shift toward a more balanced and resilient oral microbiome (Table 6, Figure 1). Participants with lower baseline diversity showed the greatest improvements, while those with higher baseline diversity maintained stable, favorable microbial profiles. These results support the product’s role in maintaining oral ecological balance and promoting beneficial commensal microorganisms.
Subjective evaluations further confirmed the product’s efficacy. Between 67% and 78% of participants reported fresher, longer-lasting breath, and 89% noted visible improvements in gum and teeth condition as well as a general sense of oral health (Table 8). The alignment of clinical, microbiological, and subjective outcomes indicates that this formula delivers both objective and perceptible benefits, making it a valuable adjunct for daily oral care routines.
In conclusion, regular use of THYMOQUIN-OCARE – BLACK SEED OIL LOZENGES TABLETS FOR ORAL CARE effectively reduces gingival inflammation, enhances oral microbiome diversity, and improves subjective perceptions of oral freshness and gum and teeth health, demonstrating its potential as a safe and effective product for maintaining oral hygiene and overall oral wellness.
- Ahmad, S., & Beg, Z. (2014). Mitigating role of thymoquinone rich fractions from nigella sativa oil and its constituents, thymoquinone and limonene on lipidemic-oxidative injury in rats. Springerplus, 3(1). DOI: https://doi.org/10.1186/2193-1801-3-316
- Alasbily, H. (2025). Probiotics in periodontal diseases: mechanisms, evidence mapping, limitations, and future directions. Cureus, 17(11), e96042.
DOI: https://doi.org/10.7759/cureus.96042 - Chaieb, K., Kouidhi, B., Jrah, H., Mahdouani, K., & Bakhrouf, A. (2011). Antibacterial activity of thymoquinone, an active principle of nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complementary and Alternative Medicine, 11(1), 29.
DOI: https://doi.org/10.1186/1472-6882-11-29 - El-Majeed, S., Bashir, M., Bulla, H., Ahmed, A., Saeed, O., Gorish, B., & Abdelmula, W. (2023). In vitro evaluation of antimicrobial activity of nigella sativa against methicillin resistant staphylococcus aureus in shendi town, sudan. International Journal of Pathogen Research, 12(6), 76-82.
DOI: https://doi.org/10.9734/ijpr/2023/v12i6255 - Waris, K., Saleem, S., Arshad, M., & Iqbal, J. (2017). A novel complementary alternative medicine: an in-vitro evaluation of efficacy of nigella sativa extract as an antibacterial agent against porphyromonas gingivalis. Annals of Punjab Medical College, 11(3), 247-251.
DOI: https://doi.org/10.29054/apmc/2017.205 - Salah, A., Doghish, Y., Abbass, S., Mansour, R., Sayed, G., Elshami, N. H., Abdel Mageed, S. S., Mohammed, O. A., Abulsoud, A. I., Bakr Zaki, M., Mosalam, E. M., Elrebehy, M. A., Alfarsi, K., & Doghish, A. S. (2025). Microbiota-based therapies in oral health and disorders. Folia Microbiologica, 70, 1217–1240.
DOI: https://doi.org/10.1007/s12223-025-01324-x - Sultan, M., Javed, S., Madkhali, O., Alam, M., Almoshari, Y., Bakkari, M. A., Sivadasan, D., Salawi, A., Jabeen, A., & Ahsan, W. (2022). Development and optimization of methylcellulose-based nanoemulgel loaded with nigella sativa oil for oral health management: quadratic model approach. Molecules, 27(6), 1796.
DOI: https://doi.org/10.3390/molecules27061796 - Ogen‐Shtern, N., Margarita, Y., & Liki, v. O. B. (2021). Antimicrobial activity by a unique composition of cold pressed nigella sativa seed (black cumin) oil. Food Science & Nutrition Research, 4(2). DOI: https://doi.org/10.33425/2641-4295.1050
- Fydrych, D., Jeziurska, J., Przekwas, J., & Kwiecińska-Piróg, J. (2025). Potential use of selected natural compounds with anti-biofilm activity. International Journal of Molecular Sciences, 26(2), 607. DOI: https://doi.org/10.3390/ijms26020607
- Polizzi, A., Donzella, M., Nicolosi, G., Santonocito, S., Pesce, P., & Isola, G. (2022). Drugs for the quorum sensing inhibition of oral biofilm: new frontiers and insights in the treatment of periodontitis. Pharmaceutics, 14(12), 2740. DOI: https://doi.org/10.3390/pharmaceutics14122740
- Jang, Y., & Mosolygó, T. (2020). Inhibition of bacterial biofilm formation by phytotherapeutics with focus on overcoming antimicrobial resistance. Current Pharmaceutical Design, 26(24), 2807-2816. DOI: https://doi.org/10.2174/1381612826666200212121710
- Shan, W., Du, F., Zhang, H., Zhang, J., Hu, X., Fan, X., & Li, W. (2025). D-histidine exhibited anti-biofilm activity against aggregatibacter actinomycetemcomitans. Microbiology Spectrum, 13(11), e0121625. DOI: https://doi.org/10.1128/spectrum.01216-25
- Dimitrova, P., Ivanova, V., Трендафилова, А., & Paunova‐Krasteva, T. (2024). Anti-biofilm and anti-quorum-sensing activity of inula extracts: a strategy for modulating chromobacterium violaceum virulence factors. Pharmaceuticals, 17(5), 573. DOI: https://doi.org/10.3390/ph17050573
- Khan, M., Çeli̇k, İ., Khan, H., Shahid, M., Shahzad, A., Kumar, S., & Ahmed, B. (2023). Antibiofilm and anti-quorum sensing activity of psidium guajava l. leaf extract: in vitro and in silico approach. Plos One, 18(12), e0295524. DOI: https://doi.org/10.1371/journal.pone.0295524
- Prabhavathi, S., Subrahmaniyan, K., Kumar, M., Gayathry, G., & Malathi, G. (2023). Exploring the antibacterial, anti-biofilm, and anti-quorum sensing properties of honey: a comprehensive review. AA, 2(3), 10-14. DOI: https://doi.org/10.51470/agri.2023.2.3.10
- Tonkin, M., Khan, S., Wani, M., & Ahmad, A. (2021). Quorum sensing – a stratagem for conquering multi-drug resistant pathogens. Current Pharmaceutical Design, 27(25), 2835-2847. DOI: http://dx.doi.org/10.2174/1381612826666201210105638
- Lemos, M., Borges, A., Teodósio, J., Araújo, P., Mergulhão, F., Melo, L., & Simões, M. (2014). The effects of ferulic and salicylic acids on bacillus cereus and pseudomonas fluorescens single- and dual-species biofilms. International Biodeterioration & Biodegradation, 86(Part A), 42-51.
DOI: https://doi.org/10.1016/j.ibiod.2013.06.011.