Article / Research Article
1Pharmaseed Ltd.
2TriNutra Ltd.
Liki von Oppen-Bezalel,
TriNutra Ltd.
28 October 2024 ; 14 November 2024
This study investigates the antiviral efficacy of ThymoQuin®(standardized black seed oil with high thymoquinone content) against the Influenza A (H1N1) virus in MDCK cells. Two test items, NSQ (ThymoQuin standardized at 3% thymoquinone) and NSQL (a blend of ThymoQuin with added lycopene, phytoene, phytofluene, and beta-carotene), were evaluated. ThymoQuin oils are cold-pressed and full-spectrum, ensuring high bioavailability and stability, which is also aided by NSQL’s carotene mixture from tomato extract. Utilizing Tamiflu as a positive control, we compare the antiviral activity of NSQ and NSQL to determine their potential as alternative or complementary treatments for influenza infections. Our findings demonstrate that both NSQ and NSQL exhibit significant antiviral activity, comparable to Tamiflu, making them promising candidates for antiviral therapy.
Thymoquinone, Influenza A (H1N1), MDCK cells, antiviral activity, bioavailability, Tamiflu, Nigella sativa, cold-pressed oil, full-spectrum oil.
Influenza A (H1N1) virus continues to pose a significant public health threat globally, necessitating the development of effective antiviral treatments. (Center for Disease Control and Prevention, (CDCP), 2023) Traditional antiviral agents like Tamiflu (oseltamivir) have shown efficacy but are also associated with resistance and side effects. (Jefferson et al., 2014). This study explores the potential of ThymoQuin, a standardized black seed oil with high thymoquinone content, as an alternative antiviral agent. Two formulations, NSQ and NSQL, were tested. Thymoquinone, a key active compound in Nigella sativa seed oil, has demonstrated various pharmacological effects, including anti-inflammatory, antioxidant, and antiviral properties. (Yuen et al., 2021).
ThymoQuin is the only standardized, cold-pressed, full-spectrum black seed oil available, with a minimum thymoquinone content of 3% and low free fatty acid content (<1.25%) that also meets the USP monograph. The proprietary formulation and cold-pressed extraction method enhance its bioavailability and stability. The inclusion of additional bioactive compounds in NSQL, such as lycopene, phytoene, phytofluene, and beta-carotene, further enhances its therapeutic potential. Thymoquinone, one of the main active compounds in Nigella sativa, has demonstrated various pharmacological effects, including anti-inflammatory, antioxidant, improved mitochondrial functions, and antiviral properties. (Munira et al., 2013).
(NSO-009-IVT Final Technical Study Report, 2023)
The composition of NSQ and NSQL was analyzed using standard analytical techniques to quantify thymoquinone and other constituents. NSQ is standardized at 3.10% thymoquinone, while NSQL contains 2.40% thymoquinone and is enriched with lycopene (0.11%), phytoene (0.19%), phytofluene (0.14%), and beta-carotene (0.32%). Both oils are cold-pressed and full-spectrum, enhancing their stability and bioavailability.
MDCK (Madin-Darby Canine Kidney) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
The antiviral activity of NSQ and NSQL was evaluated using an XTT-based cell viability assay. MDCK (Madin-Darby Canine Kidney) cells were plated in 96-well plates at a density of 5×10⁴ cells per well and allowed to attach for 16-24 hours at 37°C in a humidified atmosphere containing 5% CO2. After the cells had adhered, they were infected with the Influenza A (H1N1) virus and treated with various concentrations of NSQ and NSQL.
Following a 48-hour incubation period, the medium was discarded, and fresh culture medium containing XTT reagent, (2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide), was added to the cells. The reduction of XTT to formazan, which correlates with cell viability, was measured spectrophotometrically at 450 nm using a microplate reader. This assay allowed for the determination of the inhibitory effect of the test items on viral replication by quantifying cell viability in the presence of the virus and comparing it to controls.
Tamiflu was used as a positive control in the antiviral assays to benchmark the efficacy of NSQ and NSQL. The standard antiviral treatment allowed for a comparative analysis of NSQ and NSQL’s performance against a well-established antiviral agent.
(NSO-009-IVT Final Technical Study Report, 2023)
NSQ was found to contain 3.10% thymoquinone, while NSQL contained 2.40% thymoquinone along with lycopene, phytoene, phytofluene, and beta-carotene. Both formulations are cold-pressed and full-spectrum, contributing to their stability and enhanced bioavailability.
NSQ and NSQL demonstrated significant antiviral activity against the Influenza A (H1N1) virus in MDCK cells. The XTT-based assay indicated a dose-dependent inhibition of viral replication. At higher concentrations, both NSQ and NSQL showed comparable efficacy to Tamiflu (Surekha & Sumathi, 2016), reducing cell viability significantly:
NSQ
- High concentration (1:10,000): 76% inhibition
- Medium concentration (1:50,000): 64% inhibition
- Low concentration (1:100,000): 50% inhibition
NSQL
- High concentration (1:5000): 88% inhibition
- Medium concentration (1:25,000): 72% inhibition
- Low concentration (1:50,000): 50% inhibition
Tamiflu
- 50 uG/ml: 75% inhibition
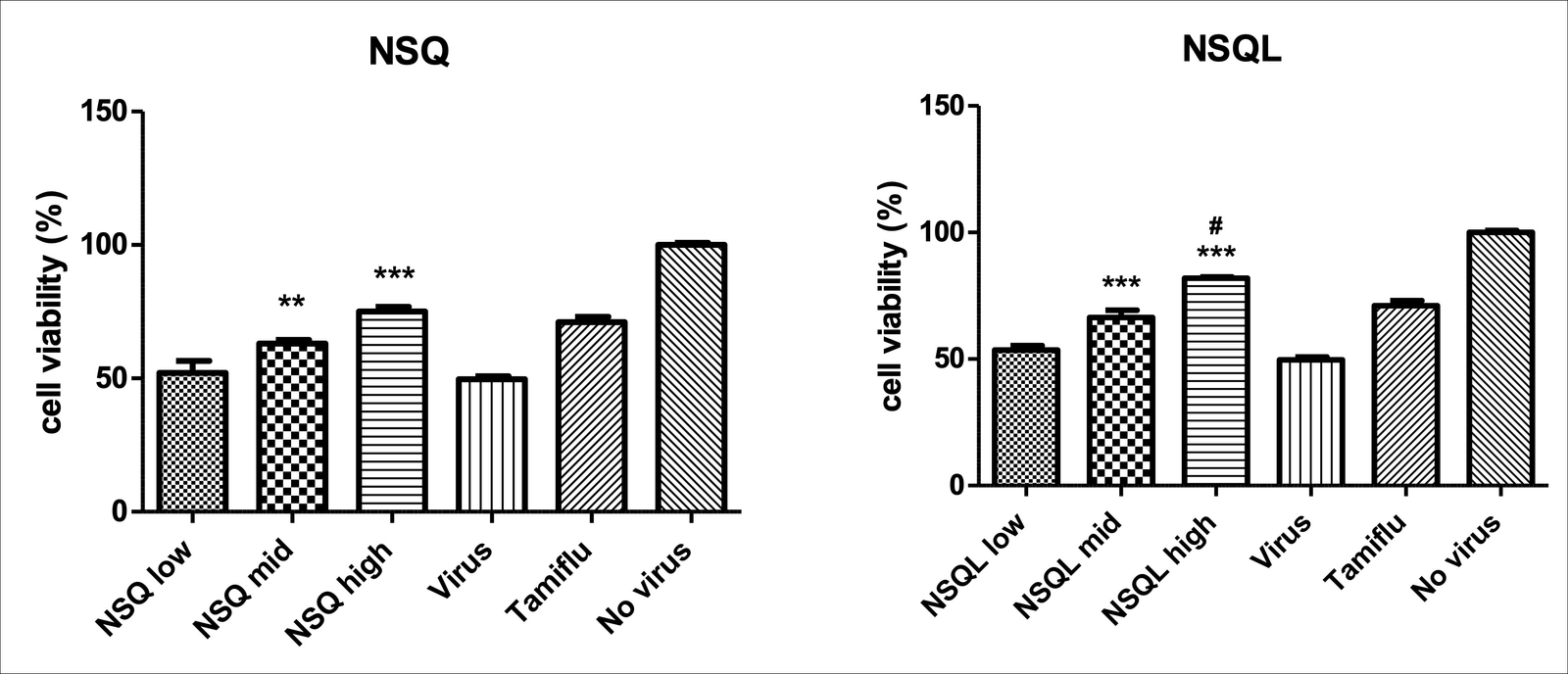 Figure 1: Dose-dependent inhibition of Influenza a (H1N1) virus replication by NSQ and NSQL in MDCK cells. This figure illustrates the percentage inhibition of viral replication at different concentrations for NSQ, NSQL, and Tamiflu.
Figure 1: Dose-dependent inhibition of Influenza a (H1N1) virus replication by NSQ and NSQL in MDCK cells. This figure illustrates the percentage inhibition of viral replication at different concentrations for NSQ, NSQL, and Tamiflu.
The results of this study demonstrate that NSQ and NSQL, both formulations of ThymoQuin, exhibit significant antiviral activity against the Influenza A (H1N1) virus, with efficacy comparable to Tamiflu. The high thymoquinone content in these formulations, particularly in NSQ, which is as active at half the dosage as of NSQL, plays a critical role in their antiviral effectiveness. The inclusion of additional bioactive compounds in NSQL, such as lycopene, phytoene, phytofluene and beta-carotene, further enhances the therapeutic potential by providing synergistic effects that support immune modulation and antioxidant activity.
A key factor contributing to the superior stability and bioavailability of NSQ and NSQL is TriNutra’s vertically integrated approach to product development. Unlike other Nigella sativa seed oil products that may rely on generic or low-thymoquinone strains, ThymoQuin is derived from proprietary strains specifically cultivated for their high thymoquinone content. This vertical integration allows TriNutra to control every aspect of the production process—from seed selection and cultivation to oil extraction and formulation—ensuring that the final product maintains consistent potency and therapeutic efficacy.
The cold-pressing technique is another critical element that contributes to the stability and bioavailability of the formulations. By preserving the full spectrum of bioactives within the oil, this method ensures that the therapeutic compounds remain intact and effective over time. The low free fatty acid content (<1.25%) in ThymoQuin further underscores the product’s stability, reducing the likelihood of oxidative degradation and enhancing shelf life.
Moreover, the proprietary strains of Nigella sativa used in ThymoQuin are a distinguishing factor that sets it apart from other black seed oil products. These strains are not only optimized for high thymoquinone content but also for their overall bioactive profile, which includes other beneficial compounds that contribute to the oil’s therapeutic effects. This specialized cultivation process ensures that the bioavailability of thymoquinone and other key compounds is maximized, which is crucial for achieving the observed antiviral effects.
The use of Tamiflu as a positive control in this study provided a benchmark for evaluating the efficacy of NSQ and NSQL. The comparable results highlight the potential of these formulations as effective alternative or complementary treatments and prevention for influenza infections. Given the challenges associated with antiviral resistance and side effects seen with traditional treatments, the natural, well-tolerated profile of ThymoQuin formulations presents a promising option for future therapeutic development.
The findings from this study underscore the potential of NSQ and NSQL as natural antiviral agents with significant efficacy against the Influenza A (H1N1) virus. The high bioavailability and stability of these formulations are directly attributable to TriNutra’s vertically integrated approach, which includes the development and use of proprietary Nigella sativa strains with optimized thymoquinone content. This vertical integration ensures that ThymoQuin® products consistently deliver potent and reliable therapeutic effects, distinguishing them from other black seed oil products on the market.
The superior stability and bioavailability of ThymoQuin® formulations, combined with their demonstrated antiviral activity, make them promising candidates for inclusion in antiviral therapy regimens. The natural composition of these oils, along with their ability to modulate immune responses and inhibit viral replication, offers a compelling alternative to traditional antiviral treatments like Tamiflu, particularly in the face of rising drug resistance and the need for safer, more natural therapeutic options.
Further research, including clinical trials, is warranted to fully establish the efficacy and safety profiles of ThymoQuin formulations in the treatment of viral infections. However, the results of this study provide a strong foundation for the continued exploration and development of ThymoQuin as a key player in natural antiviral therapy.
- Center for Disease Control and Prevention (CDCP). (2023 October 13). U.S. Influenza Surveillance: Purpose and Methods. FluView. https://www.cdc.gov/flu/weekly/overview.html
- Jefferson, T., Jones, M., Doshi, P., Spencer, E. A., Onakpoya, I., & Heneghan, C. J. (2014). Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ, 348(g2545). DOI: https://doi.org/10.1136/bmj.g2545
- Yuen, K., Alex, A., Raffaelle, M., Shen, H., Ospino, J., Bellner, L., Abraham, N. G., & Peterson, S. J. (2021). Beneficial effect of 3% Thymoquinone on stem cell-mediated improvement in immune system and anti-Inflammatory function. Journal of Food & Nutritional Sciences, 3(3), 63-74. http://www.starlingscience.com/article/beneficial-effect-of-3-percent-thymoquinone-on-stem-cell-mediated-improvement-in-immune-system-and-anti-inflammatory-function.pdf
- Munira, M., Momin, S., Kurhade, S., Khan, M., Butte, K. (2013). Nigella Sativa: Blessed Seed. International Journal of Research in Phytochemistry & Pharmacology, 3(2), 78-84. https://scienztech.org/index.php/ijrpp/article/view/852
- NSO-009-IVT Final Technical Study Report. 2023.
- Surekha, R. & Sumathi, T. (2016). An Efficient Encapsulation of Thymoquinone Using Solid Lipid Nanoparticle for Brain Targeted Drug Delivery: Physicochemical Characterization, Pharmacokinetics and Bio-Distribution Studies. International Journal of Pharmaceutical and Clinical Research, 8(12), 1616-1624. https://impactfactor.org/PDF/IJPCR/8/IJPCR,Vol8,Issue12,Article11.pdf













